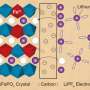
A new look at the solid-liquid interface
Interesting things happen at interfaces, and when solids meet liquids it is no exception. Understanding the complex phenomena that take place at this 'solid-liquid' interface could give us important clues about how to build better medical devices and longer-lasting batteries, but to date it has been difficult to get a handle on how chemical ions in the solution behave at this crucial juncture. Until now, that is.
A team led by UCD scientists has developed a new tool to build up a clearer picture of what is going on at this interface, and crucially, can do this on the nanoscale. The procedure, which is published in Nature Communications, stands to enable research in biological and materials science.
"The solid-liquid interface is the location of many important physical, biological and chemical processes," explains researcher Liam Collins, who is completing a Ph.D. in the Nanoscale Function Group. "If you want to understand biosystems, diseases and novel biomaterials, or processes in energy systems like batteries, you need to understand what happens at the solid-liquid interface."
What happens on the atomic level at this interface can have an impact at a more visible or macroscopic level - the way the body functions, or how quickly a battery drains, for example - so techniques that can operate on atomic-length scales can improve our fundamental understanding of materials and devices, notes Collins, who works with Dr Brian Rodriguez at UCD Conway Institute of Biomolecular and Biomedical Research.
Existing techniques, such as the atomic force microscope, already allow researchers to get a good 'view' of physical structures at the solid-liquid interface, but not how ions behave at this interface, he explains: "So we set out to join structural information with electrochemical function."
To get this multi-modal view, Collins worked with colleagues in UCD, Oak Ridge National Laboratory in the US and Taras Shevchenko Kiev National University in Ukraine to develop a technique called electrochemical force microscopy (EcFM).
The benefit of the new technique is that is allows researchers to get a clearer picture of what is going on at this key solid-liquid interface in situ rather than making measurements in air and extrapolating to liquids, explains Collins.
The scientists are now turning their attention to new materials, one being a form of ultra-thin carbon called graphene, which has applications in energy storage. "Probably the immediate improvement coming out of this EcFM technique will be a better understanding of energy systems such as double-layer capacitors and lithium ion batteries," says Collins. "If we can understand the processes on the nanoscale here, it will in turn allow us to improve the efficiency and lifetime of devices."
He also has an eye to a wide range of longer-term applications that could come from better understanding the relationship between structure and function in biological systems. "That may help us to develop in vivo batteries which harness biofuels, or to understand diseases, such as Alzheimer's disease, at a fundamental level."
Journal information: Nature Communications
Provided by University College Dublin