Scientists discover channel used by catalyst to produce ammonia, vital for food and fuel crops
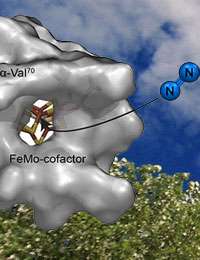
(Phys.org) —Mother Nature's helper in turning nitrogen from the air into ammonia is an enzyme called nitrogenase that uses molybdenum and iron; scientists want to learn natural catalyst's secrets and apply them to synthetic catalysts. To do so, they first need to know how the nitrogen gas reaches the heart of the catalyst. While scientists have posited long, convoluted routes, a team from Pacific Northwest National Laboratory and Utah State University discovered the actual channel the nitrogen uses. It is short and direct. They discovered the channel by identifying groups of proteins on the catalyst's surface that guard access to the metal atoms, twisting aside to allow nitrogen in.
"Our channel is the only one anyone has seen in action," said Dr. Simone Raugei, a theoretical chemist at PNNL who worked on the study.
Producing ammonia for use in fertilizer is an energy-intense reaction, known as the Haber-Bosch reaction, that requires high temperatures, around 500 degrees Celsius. The reaction consumes hydrogen gas derived from fossil fuels. Nitrogenase takes fundamentally different process to produce ammonia, and this natural reaction happens quickly at room temperature. If a synthetic catalyst could be designed that operates efficiently at room temperature and consumes available protons and electrons instead of hydrogen gas, it would greatly benefit both the agricultural and energy sectors. The discoveries made in this study provide vital information for designing such catalysts.
"We knew where the reaction happened," said Dr. Dayle Smith, a computational chemist at PNNL who conducted the study. "But, we didn't know how the nitrogen got there. Now we do."
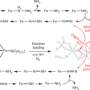
Methods: The researchers modeled molybdenum-dependent nitrogenase, a fast, efficient, and prevalent enzyme. The natural catalyst drives a reaction that turns one nitrogen molecule into two ammonia molecules. The reaction happens at the active site, buried deep inside the molecule. To access the metals at the active site, nitrogen gas must traverse channels from the surface to the active site.
Previously, scientists suggested three possible channels that directed the nitrogen to the active site. Each of these channels was long and convoluted. To determine the actual channel, the team ran submicrosecond atomistic molecular dynamics simulations using a supercomputer at EMSL. The simulations provide the location and trajectories of each atom as the enzyme catalyzes the reaction.
"Being able to see how the atoms move-you can get a lot out of that," said Smith.
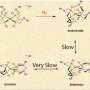
In analyzing the data, the team found the channel was short and not far away at all, but rather close to where the chemistry happens. The channel is covered by protein groups on the surface that act as flaps that open to allow the nitrogen to enter.
"Nobody could see it before because you had to have the protein moving to see it," said Smith.
The team further analyzed the viability of the channel by studying the energy required for the nitrogen to move through the catalyst. Their calculations showed that nitrogen uptake occurs along a low-energy path.
More information: Smith D, K Danyal, S Raugei, and LC Seefeldt. 2014. "Substrate Channel in Nitrogenase Revealed by a Molecular Dynamics Approach." Biochemistry 53(14):2278-2285. DOI: 10.1021/bi40131
Journal information: Biochemistry
Provided by Pacific Northwest National Laboratory