October 21, 2013 report
Light-activated medical implants: Team develops light-guiding hydrogel for cell-based sensing
(Phys.org) —A combined team of researchers from Harvard Medical School and several research institutions in Korea has developed a light-sensitive hydrogel that can be used as a cell based sensor. In their paper published in the journal Nature Photonics, the team describes their hydrogel and the ways it can be used.
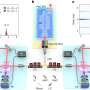
Scientists have known for some time that light can be used to trigger changes in light sensitive cells. They've also discovered that light can be used to help in visualizing biological functions in living organisms, such as mice and people. Unfortunately, in order to use such devices, light must be able to penetrate the skin, fat, muscle and other tissue in order to be useful inside the body. Now, in this new effort, the researchers have devised a means for circumventing this problem: a light-guiding hydrogel scaffolding capable of holding on to engineered cells.
The hydrogels are made of water, biopolymers and cell media—their purpose is to allow for holding onto cells or more importantly, bioengineered cells after placement in the body (of mice thus far). The engineered cells respond in desired ways when exposed to light. To allow that to happen, the researchers fed a fiber optic cable though the skin of test mice that traveled to the location of the implanted hydrogel. Light fed into the fiber cable was routed directly to the hydrogel—the cells in its scaffolding then responded according to how they'd been programmed.
In one of their experiments, the researchers used programmed HeLa cells that were caused to emit a kind of protein when exposed to light. When that protein is produced in the body, the pancreas creates more insulin. Thus, a means of controlling diabetes was shown. In another experiment, the researchers filled the hydrogel with cells that light up when exposed to a certain toxin, then after implanting the hydrogel, injected mice with the toxin. Using the fiber cable in reverse, the researchers were able to see the cells in the hydrogel lighting up in response to the presence of the toxin.
.jpg)
The hydrogels the team is working with are not yet ready for testing in people, though that is the ultimate goal. The hope is that some day soon, doctors may be able to implant a hydrogel into a person and then use it to either control a disease or to detect the presence of toxins or other chemicals that reside deep inside the body.
More information: Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo, Nature Photonics (2013) DOI: 10.1038/nphoton.2013.278
Abstract
Polymer hydrogels are widely used as cell scaffolds for biomedical applications. Although the biochemical and biophysical properties of hydrogels have been investigated extensively, little attention has been paid to their potential photonic functionalities. Here, we report cell-integrated polyethylene glycol-based hydrogels for in vivo optical-sensing and therapy applications. Hydrogel patches containing cells were implanted in awake, freely moving mice for several days and shown to offer long-term transparency, biocompatibility, cell viability and light-guiding properties (loss of <1 dB cm−1). Using optogenetic, glucagon-like peptide-1 secreting cells, we conducted light-controlled therapy using the hydrogel in a mouse model with diabetes and obtained improved glucose homeostasis. Furthermore, real-time optical readout of encapsulated heat-shock-protein-coupled fluorescent reporter cells made it possible to measure the nanotoxicity of cadmium-based bare and shelled quantum dots (CdTe; CdSe/ZnS) in vivo.
Journal information: Nature Photonics
© 2013 Phys.org