Mapping replacement of dead cells in the intestine uncovers critical stem cell pool
Stem cells play a critical role in replacing various cells within the intestine, but can also become drivers for colorectal cancer. The composition of these stem cell reservoirs has been debated. New research from Dmitry Bulavin of the A*STAR Institute of Molecular and Cell Biology has now clarified the organization of this tissue, yielding insights that should steer future cancer research.
Bulavin and co-workers had previously identified a population of '+4' cells—denoting their position within the intestinal epithelium—that appeared to contain intestinal stem cells (ISCs)2. They determined that activation of certain oncogenes rapidly kills these cells via a mechanism called apoptosis, a critical safeguard against cancerous growth. Accordingly, mice with a genetic mutation that increased the apoptotic response of these cells were also protected against intestinal tumors. However, subsequent findings from another team identified a different pool of putative ISCs, identifiable by their expression of the marker gene Lgr5.
"We could not explain the tumor-resistant phenotype of our mice using this new model," says Bulavin. "This motivated further analysis of different stem cell populations in the intestine." His team employed a technique called 'lineage tracing', which enables selective labeling of cells expressing a marker gene of interest with a fluorescent protein. Descendants of these cells become similarly labeled, allowing researchers to track the point of origin for cell populations or tissues of interest. Bulavin and co-workers then used radiation or chemical trauma to induce apoptosis, and examined the response of different cell populations to identify likely ISCs.
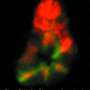
Their experiments revealed that a pool of +4 cells, identifiable by expression of the cellular marker gene Bmi1, appear to represent true ISCs. These cells are normally dormant, but sporadically replicate to replace intestinal cells lost to normal wear and tear (see image). They can also be rallied to repair more severe damage. "Massive cell death is a very powerful factor in the stimulation of these +4 ISCs," says Bulavin. "They exit quiescence and enter a proliferative state after high doses of irradiation." The previously identified pool of Lgr5-expressing cells in turn appears to consist of progenitor cells, which develop into ISCs only when Bmi1-positive ISCs are depleted.
Armed with some initial insights into how improper regulation of apoptosis affects these various cell populations, Bulavin's team is now working to understand their relevance in cancer. "We want to see how apoptosis affects the development of polyps when oncogenic mutations occur in different cellular compartments in the mouse intestine," he says.
More information: Zhu, Y., Huang, Y.-F., Kek, C. & Bulavin, D. V. Apoptosis differently affects lineage tracing of Lgr5 and Bmi1 intestinal stem cell populations. Cell Stem Cell 12, 298–303 (2013). dx.doi.org/10.1016/j.stem.2013.01.003
Demidov, O. et al. Wip1 phosphatase regulates p53-dependent apoptosis of stem cells and tumorigenesis in the mouse intestine. Cell Stem Cell 1, 180–190 (2007). www.sciencedirect.com/science/ … ii/S1934590907000641
Journal information: Cell Stem Cell