Sudy unravel mechanism critical for fungal virulence
Metallothioneins, proteins able to capture metal ions, play a major role in the virulence of Cryptococcus neoformans, a fungal pathogen which causes severe infections in immunodeficient and immunocompetent individuals (AIDS patients, transplant receivers, etc.) This is one of the main conclusions of the research published on the journal Cell, Host & Microbe, and developed by the researchers Sílvia Atrian and Anna Espart, from the Department of Genetics and the Institute of Biomedicine of the University of Barcelona (IBUB), affiliated with the campus of international excellence BKC.
Proteins which bind metal ions
Discovered in 1957 by the experts Marghoses and Vallee, metallothioneins (MT) are low molecular weight, cysteine-rich proteins. Thanks to their structure, they can bind metal ions and act as chelating agents —compounds which capture metals— to capture and distribute biologically interesting metals (copper, zinc, cadmium, quicksilver, etc.). MTs are very heterogeneous and polymorphic, and can be found in any type of organism (prokaryotes, fungi, plants, vertebrates, etc.), in which they facilitate metal detoxification processes and help to modulate the of the organism's physiological response against a lack or excess of metals.
The fight against an opportunistic fungus
Cooper has a long history as an antimicrobial agent. To capture and eliminate cooper excess is a major step forward in the progress of infections.
Previous studies identified some proteins produced by C. neoformans in response to high cooper concentrations. "This new research states for the first time that these proteins are metallothioneins; they play a critical role in virulence and colonization of the pathogen", explain Professor Sílvia Atrian, head of the Consolidated Research Group on Metallothioneins, Metallomics and Networks of Response to Metals (METMET), composed by experts from the UB and the UAB and recognised by the Government of Catalonia. The experts on bioinorganic chemistry Jordi Espín i Òscar Palacios, from the collaborating group headed by Mercé Capdevilla (UAB) and members of METMET, also participate in the study, led by Dennis J. Thiele (Duke University, USA).
The study proves that the genetic expression of C. neoformans metallothioneins is active in pulmonary infection. According to researcher Anna Espart, "when the fungus can infect lungs, macrophage cells —and other defence strategies—increase cooper concentrations to combat the infection. In a high cooper environment, the synthesis of C. neoformans metallothioneins is activated; they can capture cooper enabling then the infection to advance in a hostile environment".
In tandem repetitions: a successful evolutive strategy
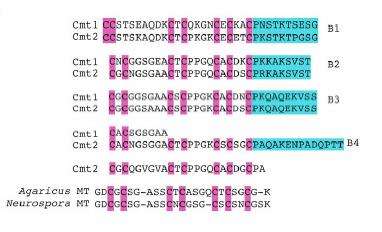
The smallest MTs, also induced by cooper, were previously found from other fungi, such as Neurospora crassa and Agaricus bisporus, fungi used in molecular biology studies. According to the experts, one of the most surprising findings is that C. neoformans MT sequences are originated by in tandem repetitions of a unit which is very similar to the one of Neurospora and Agaricus, which can bind six cooper atoms.
"MTs which show a similar modular structure to the one of Cryptococcus have been also identified in other fungal pathogens", highlights Professor Atrian. "Data points out that —she adds— C. neoformans MTs are longer and have an exceptionally high cooper binding capacity compared to other MT proteins, perhaps due to evolutionary pressure to evolve by tandem amplification. So, it is not an isolated characteristic, but an evolutive strategy of certain pathogens to successfully infect different hosts, ranging from plants to people". The expert ensures that this evolutive strategy is different from MTs' one in most multicellular organisms, "which is based on making several copies of a certain gene to synthetize proteins specialised in specific biological functions". For example, this happens in mammals, as they have four metallothionein isoforms (MT1, MT2, MT3 and MT4).
The new research, carried out with mice, shows that when MTs have been modified and are not able to bind metals, the pathogen is unable to infect host cells. "From a therapeutic point of view, results prove that any element which interferes in MT synthesis can avoid the infection development", explains the researcher Anna Espart. A better knowledge of the molecular mechanism that inhibits protein synthesis and inactivates pathogen virulence opens up new horizons in the international research on new pharmacological and therapeutic tools against cryptococcosis.
More information: www.sciencedirect.com/science/ … ii/S1931312813000681
Journal information: Cell Host & Microbe
Provided by University of Barcelona