April 30, 2013 feature
Antibiotics 2.0: The atomic structure and mechanism of mammalian host-defense peptides
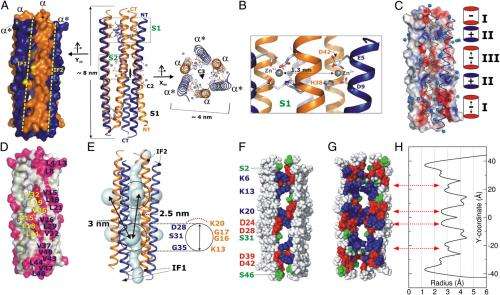
(Phys.org) —While the natural world is replete with compounds that form the basis of many disease-fighting pharmaceuticals, it is also the case that humans and other mammals produce their own host-defense peptide-derived broad-spectrum antibiotics to combat bacterial and fungal infections. By attacking microbial cell membranes, these peptides prevent bacteria from developing rapid antibiotic resistance. While over 1,700 of these peptides are known, the structural and mechanical aspects of their functional activity have remained an unanswered question. Recently, however, scientists at Max Planck Institute for Biophysical Chemistry, Max Planck Institute for Developmental Biology, The University of Edinburgh, and other instiutions1 determined the X-ray crystal structure as well as solid-state nuclear magnetic resonance (NMR) spectroscopy, electrophysiology, and molecular dynamic (MD) simulations of human dermcidin (DCD), revealing its mechanism at atomic scale. The researchers conclude that their results may lead to the peptide structure-based design of second-generation antibiotics.
Prof. Kornelius Zeth spoke with Phys.org about the challenges that he, Dr. Ulrich Zachariae, Dr. Chen Song, Dr. Bert de Groot, and his other colleagues encountered in conducted their research. "For crystallography it was very difficult to find a proper peptide source. In fact, we had to use five different peptides from different companies until we found a company, Peptide 2.0 Inc., which produced the peptide in the correct way," Zeth recalls. "However, I can't say they've modified their process – and luck also a factor, as are and several parameters which are influential aside from the peptide, such as who conducts the robotic screening, local climate and so on."
Moreover, the simulations that revealed the antibiotic mechanism of human dermcidin had their own complications. "Simulations require time and experience in order to capture the correct lipidic environment," Zeth adds. "Also, the choice of the proper MD method was critical to tracing the mechanism by which ions passed through the channel." The simulations went on for many months, he illustrates, depending on the system studied, to get a complete picture of the membrane channels. More specifically, Song notes that the production simulations took more than a year – roughly 650 days – in real time using 48 CPUs.
"Several consistency checks were carried out to ensure the validity of the MD simulations," de Groot points out. "First, the simulation parameters and setup were carefully selected to mimic the bacterial conditions as closely as possible. Second, to ensure convergence the simulations were carried out multiple times, from different starting conditions. Third, the conductance derived from the electrophysiology simulations were directly compared to measured values, showing quantitative agreement."
Another challenge, Zeth says, was conducting electrophysiology experiments in which the scientists characterized the activity of dermcidin in membranes under various conditions. "Methods applied to solve this problem are typically based on recent developments in electrophysiology. One general problem of DCD is that the peptide is very soluble in water and does not easily enter artificial membranes." in fact, this finding has also caused NMR problems when trying to verify the tilting of the channel in membranes as predicted by MD simulations. These studies were hampered by the small portion of the peptide that was observable in an integral membrane conformation.
"Peptide solubility or membrane affinity can be influenced by several parameters, such as mutations or changing membrane systems," Zeth continues. "For NMR we've seen essentially all of the peptide associated with the membrane in a horizontal manner. However, we were more interested in the peptide complex inserted in the membrane, but that only occurred ten percent or less of the time."
Zeth outlines the key insights and discoveries that helped the team address these challenges. "One important factor was that this was the first structure of an anti-microbial peptide in a channel conformation," he explains. "Another was that this peptide activity seems to be zinc-dependent – although it is not really clear what activity zinc actually causes. Also," Zeth adds, "our model, based on the molecular dynamics of ion flow through the channel, is new and unexpected, in that lateral pore openings seem to play a more important role than do terminals."
Zeth points out that their study solved the crystal structure of an AMP (antimicrobial peptide) channel for the first time in a putative channel conformation. "This result allowed a number of follow-up experiments, in particular MD simulations without assembling a rather artificial membrane pore," he explains. "In my opinion, our AMP structure is the first to be assembled on the basis of direct X-ray data. All other AMPs used for MD are assembled based on indirect data, and while these assemblies may be correct, their structural information cannot be compared to the quality of X-ray data. This allowed us to use a system that reflects the closest membrane-bound state."
Discussing how their findings articulate the comprehensive mechanism for the membrane-disruptive action of this mammalian host-defense peptide at atomistic level, Zeth notes that their findings are based on the combination of several techniques. "Starting from the crystal structure," he explains, "we modeled the ion flux, later deciding to verify the theoretical calculations and values by experimental data. We've also tried to model the tilting behavior seen in MD simulation by solid state NMR techniques, but this approach failed. Nevertheless, the overall picture, while incomplete, leads to many follow-up experiments and stimulates the AMP community regarding the development of new mechanistic views." As for the conductance calculated by MD, however, researchers we were able to reproduce these values using planar lipid membrane techniques in an error range of only 20%.
While their paper states that their results may form a foundation for the structure-based design of peptide antibiotics, Zeth says that this is rather difficult to judge. "In fact, he says, the German company BRAIN is trying to introduce DCD into pharmaceutical studies. However, if you look closer into the general applications of peptides as AMPs, it's rather frustrating. To my knowledge," Zeth acknowledges, "there are no AMP peptides sold as pharmaceuticals at the moment, although the general market of peptides – against cancer and other diseases – is still growing. The problem is the size and stability of the peptides if given orally or into the bloodstream as proteases, but. DCD is active on skin - so might be easier if it was to be introduced into creams."
Zeth then described medical properties and potential of host-defense antimicrobial peptides for comparison with traditional small-molecule antibiotics.
- Specificity: Rather unspecific. Gram-positives and negatives are concerned while with traditional antibiotics either Gram+/Gram- are treated.
- Efficacy: likely to be more efficient due to inherent molecular antibacterial properties
- Safety: Not yet determined but as these molecules come from humans they should not be toxic
- Post-xenotransplantation immune response: Currently unknown. The problem with antimicrobial peptides is protease-based instability in the bloodstream and stomach.
- Autoimmune diseases: Some peptides, such including LL-37, have severe implications in autoimmunity. In fact, the overproduction of these peptides generates a number of skin diseases, such as psoriasis. More specifically, in the case of psoriasis it seems that LL-37 overproduction can cause autoimmune diseases,2 the unresolved issue being whether the peptides are produced inside or outside the cell.
In terms of next steps in their research, Zeth mentions studies of mutations in the membrane-exposed side of the channel; changing properties, such as conductivity or membrane integration, inside the membrane to; peptide translocation via membranes; and mentions post-xenotransplantation immune response as "an idea for future applications."
Zeth notes that other areas of research might benefit from their research. "Generally our study will influence the current view of peptide channels in membranes – an important field, in particular for researchers trying to enhance this domain of knowledge to improve current peptide drugs. Also, our channel study benefits MD for further use in a variety of studies on mutations and different approaches to better characterize the channel. The channel is thereby a tool," Zeth concludes, "for testing methods and answer questions which were, so far, difficult to answer due to missing structural models."
More information: Crystal structure and functional mechanism of a human antimicrobial membrane channel, PNAS March 19, 2013 vol. 110 no. 12 4586-4591, doi:10.1073/pnas.1214739110
Related:
1Georg August University – Göttingen, University of Strasbourg, Centre National de la Recherche Scientifique, Freiburg University, University of Basque Country, Spanish Science Research Council
2Editor's Summary: Trigger for psoriasis, Nature 4 October 2007
Journal information: Proceedings of the National Academy of Sciences , Nature
© 2013 Phys.org. All rights reserved.