Titanium dioxide nanoreactor
Tiny particles of titanium dioxide are found as key ingredients in wall paints, sunscreens, and toothpaste; they act as reflectors of light or as abrasives. However with decreasing particle size and a corresponding change in their surface-to-volume ratio, their properties change so that crystalline titanium dioxide nanoparticles acquire catalytic ability: Activated by the UV component in sunlight, they break down toxins or catalyze other relevant reactions.
Now, Dr. Katja Henzler and a team of chemists at the Helmholtz Centre Berlin have developed a synthesis to produce nanoparticles at room temperature in a polymer network. Their analysis, conducted at BESSY II, Berlin's synchrotron radiation source, has revealed the crystalline structure of the nanoparticles. This represents a major step forward in the usage of polymeric nanoreactors since, until recently, the nanoparticles had to be thoroughly heated to get them to crystallize. The last synthesis step can be spared due to the special environment inside the PNIPAM network.
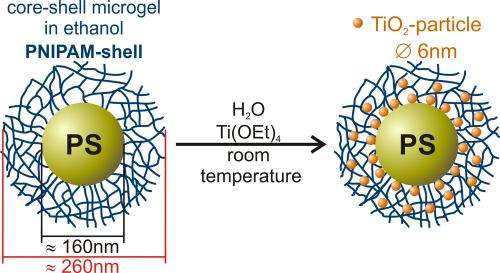
The Henzler team's polymeric nanoreactors consist of a polystyrene core surrounded by a network of PNIPAM chains. A titanium compound was added to an ethanolic solution of the polymer colloids, which did trigger the formation of small titanium dioxide particles within the PNIPAM network. The BESSY II experiments showed that the chemists were able to control the speed of these processes while at the same time affecting the quality of the nanocrystals that had formed.
Using the novel combination of x-ray microscopy and spectroscopy (NEXAFS-TXM, U41-SGM) at BESSY II, Henzler and the microscopy team were able to show that the nanoparticles are homogeneously distributed over the polymeric nanoreactors. The researchers examined their samples in a cryogenic aqueous environment, which prevents artifact formation due to sample drying. Their analysis showed that the nanoparticles have a crystalline structure. "The nanocrystals have a tetragonal anatase structure and this crystalline structure is a key to their catalytic performance. Additionally, our new analytic method allows us to control the quality of the synthesized particles so that we can optimize them for relevant applications," says Katja Henzler.
More information: Nano Letters, 2013, 13 (2), pp 824–828; DOI: 10.1021/nl3046798
Journal information: Nano Letters
Provided by Helmholtz Association of German Research Centres