Yeast protein breaks up amyloid fibrils and disease protein clumps differently
(Phys.org)—Several fatal brain disorders, including Parkinson's disease, are connected by the misfolding of specific proteins into disordered clumps and stable, insoluble fibrils called amyloid. Amyloid fibrils are hard to break up due to their stable, ordered structure. For example, α-synuclein forms amyloid fibrils that accumulate in Lewy Bodies in Parkinson's disease. By contrast, protein clumps that accumulate in response to environmental stress, such as heat shock, possess a less stable, disordered architecture.
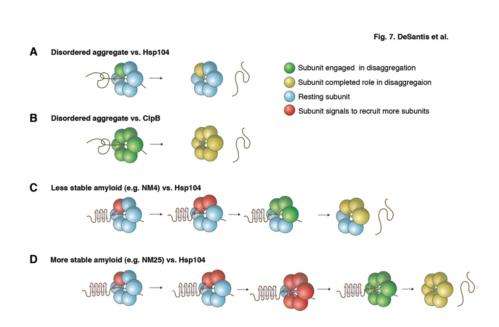
Hsp104, an enzyme from yeast, breaks up both amyloid fibrils and disordered clumps. In the most recent issue of Cell, James Shorter, PhD, assistant professor of Biochemistry and Biophysics, and colleagues from the Perelman School of Medicine, University of Pennsylvania, show that Hsp104 switches mechanism to break up amyloid versus disordered clumps. For stable amyloid-type structures, Hsp104 needs all six of its subunits, which together make a hexamer, to pull the clumps apart. By contrast, for the more amorphous, non-amyloid clumps, Hsp104 required only one of its six subunits.
Unexpectedly, the bacterial version of the Hsp104 enzyme, called ClpB, behaves differently compared to Hsp104. Bacterial ClpB uses all six subunits to break up amorphous clumps and fails to break up amyloid fibrils. Bacteria just ignore these more stable structures, whereas yeast use Hsp104 to exploit amyloid fibrils for beneficial purposes.
"One surprise is that biochemists thought that Hsp104 and ClpB hexamers worked in the same way," says first author and graduate student in the Shorter lab Morgan DeSantis. "This is not the case."
Hsp104 breaks up the protein clumps by "pulling" individual polypeptide chains through a channel that the hexamer forms at its center, recruiting more subunits to the job, as needed. Individual polypeptides emerge on the other side where they can be refolded into active structures. Remarkably, Hsp104 broke up various amyloid fibrils formed by proteins connected to Alzheimer's disease (tau and Ab42), Parkinson's disease (α-synuclein), Huntington's disease (polyglutamine), and even type II diabetes (amylin).
The bad news is that animals do not harbor their own version of Hsp104 and they do not appear to have the protein machinery to break up amyloid clumps as rapidly. But Shorter views this as a possible therapeutic opportunity: "We want to introduce Hsp104 transiently as a therapeutic clump buster and optimize Hsp104 for each type of disease protein." He is heartened by preclinical evidence that Hsp104 rescues neurodegeneration caused by α-synuclein misfolding in a rat model of Parkinson's disease. His lab is now scanning yeast cells to look for the most useful forms of Hsp104.
More information: www.sciencedirect.com/science/ … ii/S0092867412012378
Journal information: Cell
Provided by University of Pennsylvania School of Medicine