Japanese researchers realize world's first oxidation reaction with well-defined molecular alignment, spin directions
Japanese researchers developed the world’s first O2 molecular beam which enables designation of the alignment of the molecular axis and spin direction.
A research group consisting of Dr. Mitsunori Kurahashi, Principal Researcher, and Dr. Yasushi Yamauchi, Group Leader of the Spin Characterization Group, both of the Nano Characterization Unit of the National Institute for Materials Science, developed the world’s first O2 molecular beam which enables us to designate the alignment of the molecular axis and spin direction. The NIMS researchers applied this beam to the surface oxidation of silicon, and discovered that only oxygen molecules with the molecular axis nearly parallel to the surface contribute to the silicon oxidation.
Molecular oxygen (O2) is one of the most important chemical species in virtually all fields of fundamental science and materials development. O2 has an anisotropic shape, i.e., is a linear molecule, and possesses spin originating from two unpaired electrons. However, until now, there have been no experimental methods which enable us to investigate how the shape and spin of an O2 molecule influence oxidation reactions. Even though the initial oxidation of silicon has been studied in detail to understand the thermal oxidation of silicon used for fabricating gate insulator films, the origin of the particularly low initial reaction probability has not been understood well.
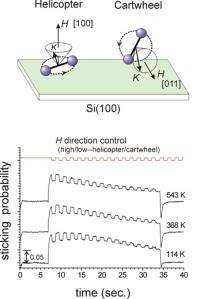
Using the magnetic hexapolar field technique, the team headed by Dr. Kurahashi developed the world’s first single quantum state-selected O2 beam which enables us to designate both the alignment of the molecular axis and the spin direction. By applying this beam to the surface oxidation of silicon, Dr. Kurahashi’s team discovered that only O2 molecules with the molecular axis nearly parallel to the surface contribute to the silicon oxidation. This research clarified that the silicon oxidation is inefficient due to the stringent geometrical requirement for the O2 axis direction, and only those molecules that satisfy a certain angular condition can participate in the reaction.
This research established a new experimental technique for analyzing the effects of the O2 alignment and the spin direction on oxidation reactions, and elucidated the origin of the inefficiency in the silicon oxidation. This technique not only enables us to scrutinize the mechanism of oxidation, but also opens up a possibility to control reactions or create new high quality materials by controlling the O2 alignment and/or spin direction.
The results were published on April 19 in the online edition of Physical Review B (Rapid Communication) of the American Physical Society.
Journal information: Physical Review B
Provided by National Institute for Materials Science