March 30, 2012 report
Researchers theorize cold compression of graphite results in new superhard carbon allotropes
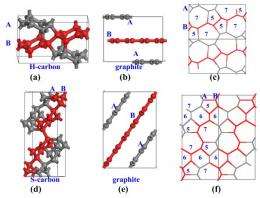
(PhysOrg.com) -- Researchers in China have used math calculations to predict that under cold compression, two new carbon allotropes may be formed. In their paper pre-published on arXiv, the team describes how the two new allotropes would have a hardness factor somewhere between graphite and diamond.
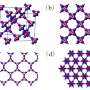
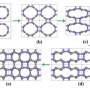
An allotrope is a substance that is essentially the same as another, with just minor differences in structure. Thus, both graphite and diamonds are allotropes of carbon. In their paper, the research team shows, via mathematical calculations, that subjecting a graphite allotrope to varying degrees of both cold and high pressure, would result in small changes to the structure, resulting in two new carbon allotropes.
Prior to this work, other researchers have theorized that applying pressure at room temperature (more than 10 GigaPascals) to graphite would also result in structural changes, creating new allotropes (M10-carbon, monoclinic M-carbon, orthorhombic W-carbon or cubic body center C4 carbon) though thus far it isn’t clear if those changes would remain in effect after the pressure is removed.
The new allotropes that theoretically would be produced by exerting pressure under cold conditions, which the team have called H-carbon and S-carbon, would also apparently be more stable than the allotropes produced without the cold, and even more stable, they say, than graphite under pressure, which means they would be more likely to survive in their compressed state after being returned to normal conditions.
By using mathematical models to predict the creation of new carbon allotropes, researchers pave the way for real world experiments to find out if the new materials would truly exist, and if so, to what purpose they might be used. New carbon allotropes would have different optical properties, such as their degree of transparency, for example or how well they reflect light, than already well understood allotropes that are already being used in real world applications,. Such properties in new allotropes, if they can be caused to persist under reasonable conditions, might lead to new and better products.
But before researchers begin trying to produce these new allotropes, more theoretical work will need to be done to see if there are others out there still waiting to be discovered.
More information: New Superhard Carbon Phases Between Graphite and Diamond, arXiv:1203.5509v1 [cond-mat.mtrl-sci] arxiv.org/abs/1203.5509
Abstract
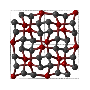
Two new carbon allotropes (H-carbon and S-carbon) are proposed, as possible candidates for the intermediate superhard phases between graphite and diamond obtained in the process of cold compressing graphite, based on the results of first-principles calculations.Both H-carbon and S-carbon are more stable than previously proposed M-carbon and W-carbon and their bulk modulus are comparable to that of diamond. H-carbon is an indirect-band-gap semiconductor with a gap of 4.459 eV and S-carbon is a direct-band-gap semiconductor with a gap of 4.343 eV. S-carbon is even more stable than the Z-carbon which is the most table carbon phase proposed recently. The transition pressure from cold compressing graphite is 10.08 GPa and 5.93 Gpa for H-carbon and S-carbon,respectively, which is in consistent with the recent experimental report.
via Arxiv Blog
© 2012 PhysOrg.com