April 22, 2011 feature
New carbon allotrope could have a variety of applications
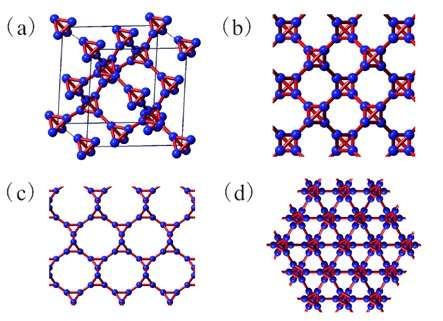
(PhysOrg.com) -- Carbon comes in many different forms, and now scientists have predicted another new form, or allotrope, of carbon. The new form of carbon, which they call T-carbon, has very intriguing physical properties that suggest that it could have a wide variety of applications.
The scientists, Xian-Lei Sheng, Qing-Bo Yan, Fei Ye, Qing-Rong Zheng, and Gang Su, from the Graduate University of Chinese Academy of Sciences in Beijing, China, have published their study on the first-principles calculations of T-carbon in a recent issue of Physical Review Letters.
Allotropes are formed when the atoms in a substance that contains only one type of atom are arranged differently. Although many substances have multiple allotropes, carbon has the greatest number of known allotropes. The three best-known carbon allotropes are amorphous carbon (such as coal and soot), graphite, and diamond. Since the 1980s, scientists have been synthesizing newer allotropes, including carbon nanotubes, graphene, and fullerenes, all of which have had a significant scientific and technological impact.
With more recent advances in synthetic tools, scientists have been investigating a wide variety of new – and sometimes elusive – carbon allotropes. In light of these investigations, Sheng, et al., write in their study that it appears that we might be entering the era of carbon allotropes.
Here, the scientists explained how to obtain a new carbon allotrope by substituting each carbon atom in diamond with a carbon tetrahedron (hence the name “T-carbon”). They were inspired by the substitution of each carbon atom in methane with a carbon tetrahedron, which forms tetrahedrane.
“[Our study] adds a possible new allotrope of carbon with amazing properties,” Su told PhysOrg.com. “T-carbon has bond angles different from graphite and diamond, but the interesting structure is still quite stable and has the same group symmetry as diamond, thereby widening people’s vision and knowledge on carbon bonding.”
Each unit cell of the T-carbon structure contains two tetrahedrons with eight carbon atoms. As the scientists’ calculations showed, T-carbon is thermodynamically stable at ambient pressure and is a semiconductor. T-carbon is one-third softer than diamond, which is the hardest known natural material. The new carbon allotrope also has a much lower density than diamond, making it “fluffy.”
The scientists also calculated that T-carbon has large interspaces between atoms compared to other forms of carbon, which could make it potentially useful for hydrogen storage. In addition, the unique physical properties of this new carbon allotrope make it a promising material for photocatalysis, adsorption, and aerospace applications.
“We believe that, if obtained, T-carbon is so fluffy that it can be used to store hydrogen, lithium, and other small molecules for energy purposes,” Su said. “It can be used as photocatalysis for water-splitting to generate hydrogen, or as an adsorption material for environmental protection. As it has very low density but a high modulus and hardness, it is quite suitable for aerospace materials, sports materials like a tennis racket, golf club, etc., and cruiser skin, and so forth.”
The scientists also noted that T-carbon could have astronomical implications as a potential component of interstellar dust and carbon exoplanets.
“There is a long-standing puzzle in astronomy known as the ‘carbon crisis’ in interstellar dust,” Su said. “Observations by the Hubble telescope revealed that the carbon budget in dust is deep in the red, and there is not sufficient carbon in dust to account for the light distortions.”
In addition, the exoplanet WASP-12b has recently been found to have a large amount of carbon, making it the first carbon-rich exoplanet ever discovered. Since the structure of the carbon in WASP-12b is still unclear, T-carbon might also be one of possible candidates for this carbon planet.
To investigate T-carbon further, the researchers would like to synthesize the new allotrope in the lab, although they say that this would likely be very difficult.
“A synthesis of T-carbon in the lab poses a great challenge for materials scientists and chemists,” Su said. “We suggest the following ways: using the CVD technique under a negative pressure environment; detonation on diamond or graphite; crystallization of amorphous tetragonal carbon; or stretching cubic diamond under extremely large strength.”
More information: Xian-Lei Sheng, et al. “T-Carbon: A Novel Carbon Allotrope.” Physical Review Letters 106, 155703 (2011). DOI: 10.1103/PhysRevLett.106.155703
Copyright 2010 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.



















