Longer-lasting chemical catalysts
Metal-based chemical catalysts have excellent green chemistry credentials—in principle at least. In theory, catalysts are reusable because they drive chemical reactions without being consumed. In reality, however, recovering all of a catalyst at the end of a reaction is difficult, so it is gradually lost. Now, chemists can retain, retrieve and reuse metal catalysts by trapping them with a polymer matrix, thanks to recent work by Yoichi Yamada at the RIKEN Advanced Science Institute, Wako, Yasuhiro Uozumi at RIKEN and Japan’s Institute for Molecular Science and Shaheen Sarkar, also at RIKEN.
Attaching metal catalysts to an insoluble polymer support, which is recoverable at the end of a reaction by simple filtration, is far from a new idea. Traditionally, chemists attached their metal catalyst to an insoluble polymer resin. However, the metal invariably leached out of the polymer over time so the catalysts were still slowly lost.
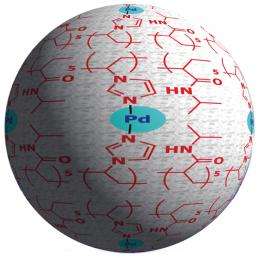
Yamada and his colleagues’ approach, in contrast, integrated the metal into the polymer matrix, which trapped it much more effectively. The researchers achieved this level of integration by starting with a soluble polymer precursor instead of an insoluble resin. This material contains imidazole units, a chemical structure known to bind strongly to metals such as palladium (Fig. 1). An insoluble composite material formed only after the researchers added palladium to the mixture because it causes the imidazole units to self-assemble around atoms of the metal—a process that they call ’molecular convolution’.
Scanning electron microscopy revealed that the resulting polymer–palladium globules ranged from 100 to 1,000 nanometers in diameter, which aggregated into a highly porous structure reminiscent of a tiny bathroom sponge. “This sponge-like insoluble material can easily capture substrates and reactants from the solution, which readily react with metal species embedded in the sponge,” says Yamada.
The researchers showed that the catalyst is highly active as well as reusable; it is the most active catalyst yet reported for a carbon–carbon bond-forming reaction known as an allylic arylation. They also reused the catalyst multiple times with no apparent loss of activity, and detected no leaching of palladium from the polymer into the reaction mixture.
Yamada and colleagues are now developing a range of composite catalysts incorporating different metals that can catalyze many other kinds of reactions. “These extremely highly active and reusable catalysts will provide a safe and highly efficient chemical process, which we hope will be adopted for industrial chemical process,” Yamada says.
More information: Sarkar, S.M., et al. A highly active and reusable self-assembled poly(imidazole/palladium) catalyst: allylic arylation/alkenylation. Angewandte Chemie International Edition 50, 9437–9441 (2011) DOI: 10.1002/anie.201103799
Journal information: Angewandte Chemie International Edition
Provided by RIKEN