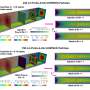
Platinum-rich shell, platinum-poor core
Hydrogen fuel cells will power the automobiles of the future; however, they have so far suffered from being insufficiently competitive. At the University of Houston, Texas, USA, a team led by Peter Strasser has now developed a new class of electrocatalyst that could help to improve the capacity of fuel cells. The active phase of the catalyst consists of nanoparticles with a platinum-rich shell and a core made of an alloy of copper, cobalt, and platinum. This catalyst demonstrates the highest activity yet observed for the reduction of oxygen.
Hydrogen fuel cells are a tamed version of the explosive reaction that occurs between oxygen and hydrogen gases to form water. To allow the reaction to proceed gently and the energy released to be tapped in the form of an electrical current, the reactants are separated within the fuel cell, and each half-reaction occurs in its own chamber. In one half-cell, oxygen takes up electrons from an electrode (reduction); in the other, hydrogen gas gives up electrons (oxidation). The cells are linked by a polymer electrolyte membrane, across which exchange occurs.
To get the reaction to proceed, the electrodes must be catalytic. For decades, the material of choice for the electrode in the oxygen half-reaction has been the precious metal platinum. Now, Strasser and his team have developed a new material, an alloy of platinum, copper, and cobalt that is deposited onto carbon supports in the form of nanoparticles. The active catalytic phase is formed in situ: when a cyclic alternating current is applied to the electrode, the less precious metals, especially the copper, on the surface of the nanoparticles separate from the alloy. This process results in nanoparticles with a core made of the original copper-rich alloy and a shell containing almost exclusively platinum.
“The oxygen-reducing activity of our new electrocatalytic material is unsurpassed—it is four to five times higher than that of pure platinum. In addition, we have demonstrated how to incorporate and activate this material in situ in a fuel cell,” says Strasser.
The observed increase in surface area of the nanoparticles is not enough to explain the increased activity. Strasser suspects that special altered structural characteristics of the surface play a role. Although the surface consists mostly of platinum, the distances between the platinum atoms on the particle surface seem to be shorter than those in pure platinum. This compression can be stabilized by the alloy core, which shows even shorter Pt-Pt distances because of the presence of copper and cobalt.
In addition, the copper-rich core seems to influence the electronic properties of the platinum shell. Theoretical calculations have suggested that the oxygen can thus bind optimally to the particle surface, allowing it to be more easily reduced.
Citation: Peter Strasser, Efficient Oxygen Reduction Fuel Cell Electrocatalysis on Voltammetrically Dealloyed Pt-Cu-Co Nanoparticles, Angewandte Chemie International Edition, doi: 10.1002/anie.200703331
Source: John Wiley & Sons