Molecular features of the circadian clock system in fruit flies
Studies of mutant fruit flies reveal how both photoreceptors and the visual system influence circadian clock neurons in response to changing light-dark cycles. The results are reported in the Journal of Neuroscience give new insights into sleep disorders including jet-lag.
Our bodies can respond to shifts in day and night patterns by reprogramming our so-called 'circadian clock' – a molecular system which responds to light-dark cycles (LD cycles). Now, Taishi Yoshii and Kenji Tomioka at the Graduate School of Natural Science and Technology, Okayama, in collaboration with scientists in Germany, have revealed how protein photoreceptors called 'cryptochromes' (CRY), together with the visual system, influence circadian clock neurons in Drosophila, or fruit flies.
Circadian clock neurons can be divided into two groups; morning neurons (M) and evening neurons (E). CRY proteins are expressed in most clock neurons and send signals in response to light, but the specific role of CRY in different types of clock neurons is unclear.
Yoshii and colleagues generated mutant fly-lines, some without CRY and others expressing CRY in different neuron subsets. They also wanted to determine the influence of the eyes and visual system signals on the neurons. Their aim was to test the flies' ability to synchronise to changes in LD cycles, a process known as LD entrainment.
They exposed the flies to an 8-hour delay in the 16-hour/8-hour LD-cycle. Control flies responded to the shift within a day, but those flies without CRY, and without eyes, were incapable of any LD entrainment. Mutant flies with E neurons were able to re-entrain, but their response was slow. The team discovered this entrainment ability was due to a molecular cycling process involving a protein called par domain protein 1 (PDP1), which is triggered by the visual input pathways, independent of CRY.
When the team then expressed CRY in the E neurons the LD entrainment process sped up considerably. If CRY was expressed only in M neurons, no LD entrainment occurred. The results indicate that CRY expression in E neurons is important to LD entrainment and that molecular cycling of PDP1 supports this process.
Cryptochromes
Circadian clock neurons respond to signals from a class of proteins called 'cryptochromes' (CRY), which are found in animals and plants. The CRY protein is photoreceptive; when exposed to blue light, CRY undergoes conformational changes, allowing it to bind to a circadian clock protein called TIMELESS (TIM). This action triggers the degradation of TIM, thereby resetting the body's circadian clock.
Previous research has shown that Drosophila (fruit flies) are highly light-sensitive – TIM degradation occurs even after exposure to a short, weak light pulse during darkness. However, how CRY and the visual system help the body to respond to changes in light-dark cycles (so-called LD entrainment) has not been fully explored.
Methodology
To determine whether separate clock neurons have different functions in CRY-dependent light responses, the researchers generated various Drosophila fruit fly lines, with different CRY expression in individual subsets of clock neurons. They also used control flies with no alterations, null-CRY lines to examine the effect of knocking out CRY altogether, and flies with null-CRY and no eyes, to examine the influence of the visual system in the LD entrainment process. The team also analyzed par domain protein 1 (PDP1), a protein important for the molecular mechanism of the circadian clock.
They exposed all the different fly groups to an 8-hour shift in LD cycle. Control flies shifted their circadian clock within a day. The mutant flies exhibited different patterns of LD entrainment, depending on CRY expression and visual inputs.
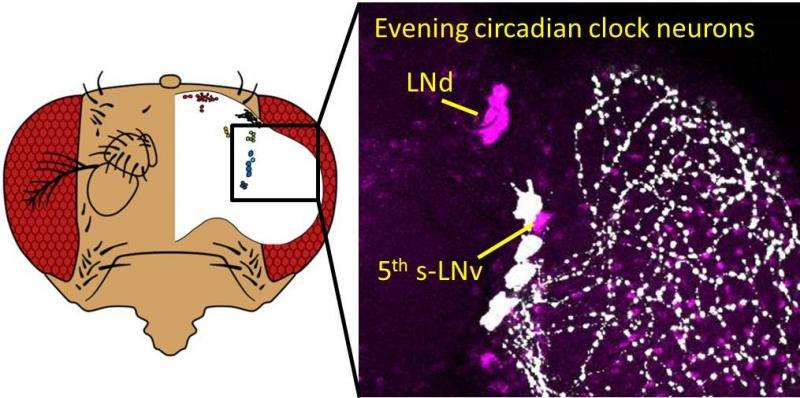
They found PDP1 worked independently of CRY to help in LD entrainment, but only in evening (E) neurons. Null-CRY E neurons were still capable of LD entrainment, but it was very slow. Reinstating CRY into two particular E neurons (fifth s-LNv and LNd), sped up the process considerably.
CRY expression in E neurons is particularly important for the rapid adjustment of the circadian clock in the event of LD cycle changes. Further work is needed to understand how morning (M) neurons factor in the LD entrainment of E neurons, and whether M cells (and another subset of neurons called PDF neurons) can contribute to LD entrainment in the absence of CRY.
More information: "Cryptochrome-dependent and -independent circadian entrainment circuits in Drosophila." J Neurosci. 2015 Apr 15;35(15):6131-41. DOI: 10.1523/JNEUROSCI.0070-15.2015
Journal information: Journal of Neuroscience
Provided by Okayama University


















