May 5, 2015 report
Controlling the internal structure of mitochondria
(Phys.org)—One might think of mitochondria as devices for transporting electrons to their lowest energy state. Little bags of finely-tuned respiratory chain subunits which combine electrons extracted from food with oxygen, and ultimately excrete them as water. Others might justifiably fancy mitochondria tiny bundles of geometry. Their folded inner membranes pegged with various proteins complexes like the rolls of candy button paper we might have ate as kids. Actually mitochondria are both enzyme bags and geometrical objects: the latest research tells us that it is proteins which create the complex inner membrane geometry specific to each kind of mitochondria, and in turn, it is their precise geometry which permits the respiratory proteins to create useful work in the first place.
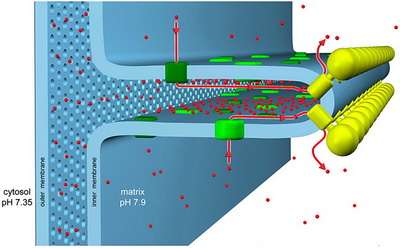
Perhaps the more difficult question here is which geometries correspond to which mitochondrial behaviors. The possible behaviors obviously include not just making ATP, but also everything from fatty acid oxidation, to synthesizing steroids, pigments, and other eclectic essentials for the host cell and organism. The possible membrane geometries, much like the various creatively-named models for the generation complex lipid membranes of myelinated nerves, come with descriptive brands like the original 'infolding baffle model' and the more modern 'crista junction' models. Crista junctions are the proteinaceous contacts made a specific critical points between the inner and outer membranes. These various folds transition in various contexts between tubular, lamellar, and helically wound sheets ( particularly common in certain protists).
A couple of papers from the past week have reported on the roles of some of the more important proteins in shaping membranes. Of note, one published by Polish and German researchers in The Journal of Biological Chemistry found a critical new protein that controls formation of the so-called MICOS system (mitochondrial contact site and cristae organizing system). This protein, Cox17, was already known for its role as a chaperone in the assembly of respiratory complex IV on the inner membrane of mitochondria. Known as cytochrome c oxidase (COX), this is the terminal respiratory complex in the whole chain—the one that hands off the electrons to oxygen. Among other amenities, Cox17 has a series of critical cysteines which appear to involved in recruiting copper to the business end of the mitochondrially encoded COX subunits.
In addition to defining a key role for Cox17 in establishing the MICOS, the researchers found that copper itself may regulate the interaction between the two. Truth be told, that point is probably not a very big deal. The MICOS seems to be a fairly sensitive assembly—if just one of its six protein subunits is missing the membrane contact points will detach. The MICOS works together with the crista junctions to maintain the deeply fissured profile of the crista. You might asking yourself about now, what exactly is the point of this peculiar clefted geometry? For that we need to look at the heavy hitter of mitochondrial proteins, the F1F0-ATP synthase. This is the guy that gets all the hard-core creationists up in a bunch for no apparent reason other than because it is simply that cool.
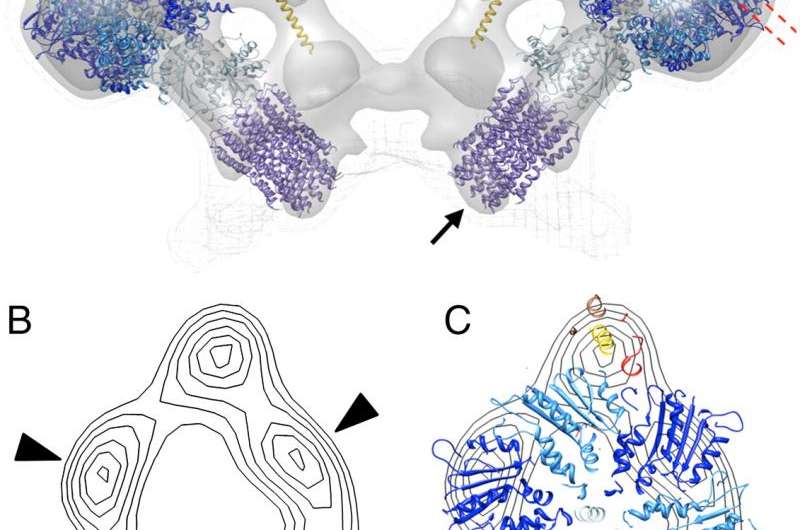
Several researchers have previously found that this 600-kDa ATP-generating monster forms dimers that localize to the highly curved wells in the deepest recesses of the crista. These dimers (and sometimes higher order multimers), are found in everything from yeast to mammals, and align themselves in rows forming a 'ridged ribbon' across much of the mitochondria. In cross section, the dimers are in contact at an angle of about 90 degrees (between the long axis of the central symmetry axis of the sythase turbine), which gives them the rough appearance of a V-8 engine. Now, the method to the madness here is that the cristae can act as 'proton traps': the respiratory chain complexes form the proton sources, and the ATP synthases form the proton sinks at the apex of each local crista compartment. Under proton limited conditions the optimal flow of ions set up in this geometry would then allow for efficient ATP synthesis.
It is not yet understood what drives synthase dimerization. It has been suggested that the spontaneous formation of dimer rows is driven more by the reduction in the membrane elastic energy than by direct protein contacts. Depending on which ATP synthase subunits are deleted, different experimental effects on mitochondrial geometry can be acheived. Deletion of subunits 'E' or 'G', which control dimerization, result in layers of onion-like inner membranes instead of the typical cristae. In some cases, the cristae are balloon-shaped and the lone synthase monomers take on a random distribution. Other research has drawn parallels between certain kinds of cristae geomtry and optimization for different kinds of metabolic activites like for example synthesis of steroids. In this case the convential wisdom is that steroid generating mitochondria are presumed to have higher crista tubularity while more typical mitochondria retain more lamellar crista instead.
Perhaps more intriguing is the recent observation in fruit flies that while ATP synthase plays a key role in germ cell differentiation, that role is completely independent of oxidative phosphorylation. In other words, when any one of ATP sythase's 13 key proteins were blocked, the manipulation stalled egg development, however, blocking other enzymes required for ATP production did not. Each organ develops it's own preferred style of mitochondria, but it may be the retina which provides the best place to try to answer the 'how and the why' geometry is optimized according to cell type and function.
Not only can a stimulus be precisely controlled and oxygen consumption easily measured for the retina, but the precise anatomical differences of rods verses cones are now known in great detail. For example, research by Marc Ellisworth has defined the connectivity and tubularity in crista structure for mitochondria found in the inner segments of photoreceptors, and correlated it with their known spiking characteristics and presumed signalling efficiency. In asking basic structure-function questions as we have before for whether a neuron should fire or not fire—ie. polarize or hyperpolarize in response to some event— like light, the costs and benefits associated with specific mitochondrial geometeries should not be ignored.
It may be a small insight to appreciate, for example, that 'rods are metabolically less costly than cones because cones don't saturate in bright light and also use more ATP in their downstream signalling pathways,' but to be able to see that directly in the mitochondria, through nothing but bare membrane geometry is a good beginning.
More information: Cox17 is an auxiliary factor involved in the control of the mitochondrial contact site and cristae organizing system, The Journal of Biological Chemistry, First Published on April 27, 2015, DOI: 10.1074/jbc.M115.645069 . www.jbc.org/content/early/2015 … 645069.full.pdf+html
Journal information: Journal of Biological Chemistry
© 2015 Phys.org