April 10, 2015 report
Inorganic nanowire follows the crystal structure of its graphene template
(Phys.org)—Graphene, a two-dimensional form of carbon, has many properties making it uniquely suited for nanodevices. For one, even though it is comprised of a network of carbon atoms, it displays extraordinary conductivity through its π-electron network. Additionally, graphene is an inexpensive, flexible substrate, making it a practical option for device construction. Many groups are interested in ways to align nanomaterials on graphene surfaces rather than functionalizing graphene, which changes some of graphene's desirable properties.
A team of researchers from the University of Tokyo, the Japan Science and Technology Agency, the University of California at Berkeley, the Ulsan National Institute of Science and Technology, Harvard University, Konkuk University, and the Lawrence Berkeley National Laboratory have discovered that gold(I) cyanide (AuCN) nanowires will assemble on pristine graphene under mild conditions. They determined that these nanowires spontaneously align with graphene's zigzag lattice, allowing for studies on graphene's structural nature as well as for the controlled design of inorganic nanostructures. Their work appears in Nature Nanotechnology.
One of the difficulties with incorporating inorganic molecules onto graphene is that graphene is chemically inert. Most efforts to produce an inorganic layer onto a graphene substrate involve either using graphene that has defects or reacting with the edges of a graphene ribbon. This study is unique in that nanowires formed on pristine graphene. Importantly, studies confirmed that the graphene remained pristine even after the nanowires formed. The nanowires were removed using a basic solution, yielding pristine graphene. Furthermore, additional studies with various types of carbon surfaces showed that the AuCN nanowires preferentially grow on pristine graphene surfaces.
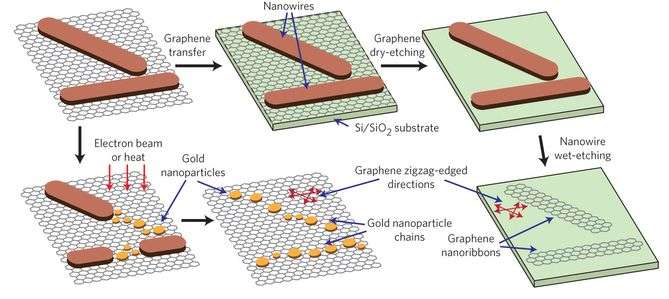
Synthesis of the AuCN nanowires was conducted under relatively mild conditions. Typically, this type of inorganic reaction in which a compound is reacted onto a substrate like graphene, is done using chemical vapor deposition. Chemical vapor deposition is done under harsh temperature and pressure conditions. Lee et al. report a synthesis in which single-layer graphene and solid gold are placed in an aqueous solution of 250 mM of ammonium persulfate at room temperature for 17 hours. The gold can be either gold nanoparticles or a gold microstructure, depending on the goals of the reaction. The acid oxidizes the gold to form nanowires. The graphene serves as a substrate for the nucleation and growth of the nanowires.
Characterization studies proved that the nanowires were composed exclusively of AuCN. Furthermore, the AuCN nanowires form a nanoribbon structure on the graphene surface in such a way that they are analogous to the graphene zigzag lattice structure. This is a key finding because the characteristics of the graphene lattice can be studied by looking at the orientation of the AuCN nanoribbons. Usually studying graphene's lattice structure requires special sample preparation and substrate requirements that can be time consuming. However, by looking at the AuCN nanoribbon properties using a technique such as scanning electron microscopy, which requires minimal sample preparation, one can more easily discern graphene's grain boundaries and other characteristics.
Because the nanowires will follow the graphene lattice structure, Lee et al. demonstrated that one could control the orientation of nanostructures. They were able to fabricate high quality graphene nanoribbons that follow a particular lattice orientation. They were also able to fabricate gold nanoparticle chains that were aligned with the graphene's zigzag lattice direction.
Because of the uniquely inert conditions for this reaction, Lee et al. conducted first-principle calculations to understand what promoted this substrate-induced nanowire formation, which may provide clues to developing a general mechanism for making nanomaterials in inert conditions.
They found that AuCN maintained its hexagonal crystal structure and graphene maintained its sp2 carbon structure. The interlayer difference between the AuCN crystals and the graphene sheet is almost the same as the interlayer difference between Au(1 1 1) and graphene. This suggests that the primary interaction is between the graphene and the gold atom in AuCN. However, the binding energy for AuCN on graphene is much higher than for Au(1 1 1), suggesting that that graphene's π electrons interact with the electron-poor gold in AuCN. This unique π interaction may be the impetus behind the spontaneous binding between the nanowires and graphene, and may be a property that can be used for constructing other nanomaterials.
Overall, Lee et al. demonstrated a facile synthesis of graphene template AuCN nanowires that spontaneously align with pristine graphene's zigzag lattice. This allows for better characterization of graphene's crystal properties as well as controlling the orientation of fabricated nanomaterials. The interaction between the π electrons and the gold atom in AuCN without disturbing graphene's carbon network is a unique interaction that may be exploited for further studies in constructing nanodevices.
Publication details
"Graphene-templated directional growth of an inorganic nanowire" Nature Nanotechnology, DOI: 10.1038/nnano.2015.36
Abstract
Assembling inorganic nanomaterials on graphene is of interest in the development of nanodevices and nanocomposite materials, and the ability to align such inorganic nanomaterials on the graphene surface is expected to lead to improved functionalities, as has previously been demonstrated with organic nanomaterials epitaxially aligned on graphitic surfaces. However, because graphene is chemically inert, it is difficult to precisely assemble inorganic nanomaterials on pristine graphene. Previous techniques based on dangling bonds of damaged graphene, intermediate seed materials and vapour-phase deposition at high temperature have only formed randomly oriented or poorly aligned inorganic nanostructures. Here, we show that inorganic nanowires of gold(I) cyanide can grow directly on pristine graphene, aligning themselves with the zigzag lattice directions of the graphene. The nanowires are synthesized through a self-organized growth process in aqueous solution at room temperature, which indicates that the inorganic material spontaneously binds to the pristine graphene surface. First-principles calculations suggest that this assembly originates from lattice matching and π interaction to gold atoms. Using the synthesized nanowires as templates, we also fabricate nanostructures with controlled crystal orientations such as graphene nanoribbons with zigzag-edged directions.
Journal information: Nature Nanotechnology
© 2015 Phys.org





















