March 24, 2015 report
New mass spectrometry technique studies kinetics of fast reactions
(Phys.org)—As chemical reactions proceed, the reactants combine to form intermediates and those short-lived intermediates eventually become products. Reaction kinetics is concerned with how long it takes for the reaction mechanism, from reactants to intermediates to products, to progress. Often, it is the intermediates that provide clues to what pathway the reaction follows, but these intermediates are typically difficult to study in fast reactions.
In order to study reaction intermediates of fast reactions, scientists need to address two problems: 1) the time it takes to mix two reactants in bulk solutions, or the diffusion-limiting mixing times, and 2) finding a method that can analyze reaction components microseconds after the reaction begins. Jae Kyoo Lee, Samuel Kim, Hong Gil Nam, and Richard N. Zare from Stanford University and the Institute for Basic Sciences and DGIST and in Daegu, Republic of Korea developed a new method that overcomes these two limiting factors for analyzing fast reactions using a combination of a microdroplet fusion technique and mass spectrometry. Their research appears in the Proceedings of the National Academy of Sciences.
Prior studies have used fused microdroplets as a way to mix reactants in several hundred microseconds. It is possible to get even faster mixing times by making the microdroplets smaller and colliding them at a faster speed. While this technique has been used for fast mixing times, it has never been used for kinetic measurements. Lee et al developed a droplet generation and fusion platform and combined it with mass spectrometry, which can detect small amounts of substance quickly, to investigate reaction kinetics at on the microsecond scale.
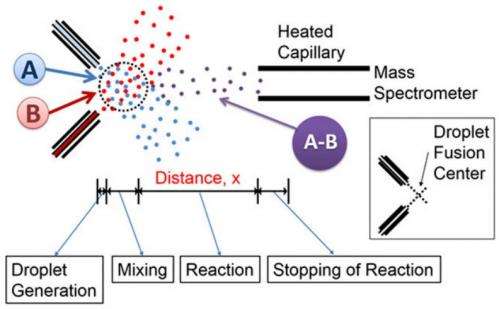
In order to obtain data on how the reaction is progressing, the authors need to ensure that the mass spectrometer is measuring the components of the reaction at a various time points as the reaction progresses. Other studies had shown that many liquid reactions stop progressing once they enter the mass spectrometer inlet and become gaseous ions. If this is the case, then varying the distance between microdroplet fusion and the mass spectrometer inlet would make reaction progress a function of length. With knowledge of the average velocity of the droplet, these measurements can then be converted to a function of time.
To test their technique, Lee et al. first looked at pure water droplets. They used a high-speed camera to characterize droplet generation, fusion, and velocity. They determined that evaporation of the pure water droplets was negligible, maintaining nearly constant droplet sizes. They were able to make droplets on the order of 13 micrometers and 93% of fusion occurred approximately within 500 micrometers from the center point of combination. This defined the distance for the start of the reaction time for subsequent studies.
They, then, wanted to test if the reaction stops at the inlet and whether they can discern rate data from a reaction with a known kinetic profile. They used 2, 6-dichlorophenolindophenol (DCIP) and ascorbic acid, which have been extensively studied to measure reaction rates in the liquid phase. This reaction behaves as a pseudo-first-order system, provided that one reagent is in excess concentration compared to the other. Their study confirmed that the reaction stopped upon entering the heated inlet of the mass spectrometer. They also found that droplet mixing was nearly complete at 0.7mm with a measured rate constant of approximately 1.0 x 105 s-1. This rate is much faster than what has been measured in bulk solution, indicating differences in chemical behavior in bulk solution verses a droplet.
The next test was to see if their technique could study the kinetics of protein unfolding. Mass spectrometry has been used to provide information on protein folding by looking at changes in the charged states, but if a protein undergoes structural changes very quickly, a slower technique would miss the initial intermediate states. The authors used cytochrome c as their model system. It unfolds in acid. They observed the typical states for cytochrome c unfolding, but they also observed additional intermediate charge states occurring on the microsecond scale.
Finally, Lee et al. looked at the hydrogen-deuterium exchange rate in bradykinin, a peptide with nine amino acids. They observed a gradual increase in mass as deuterium replaced hydrogen in the peptide. This technique is often used to discern higher-order structural changes in proteins and peptides because certain hydrogens are more prone to deuterium change than others. They observed a rapid deuterium exchange until approximately 17 microseconds, followed by three slow deuterium exchanges at 30 microseconds.
While the reactions that occur at the microdroplet scale do not precisely mimic those in bulk solution, they still progress through the same major reaction pathway. This experiment has shown that microdroplet mixing coupled with mass spectrometry may be a robust tool for discerning the intermediate components and kinetics of fast reactions in the liquid phase.
More information: "Microdroplet fusion mass spectrometry for fast reaction kinetics" Jae Kyoo Lee, Samuel Kim, Hong Gil Nam, and Richard N. Zare, PNAS, www.pnas.org/content/early/2015/03/12/1503689112
Abstract
We investigated the fusion of high-speed liquid droplets as a way to record the kinetics of liquid-phase chemical reactions on the order of microseconds. Two streams of micrometer-size droplets collide with one another. The droplets that fused (13 μm in diameter) at the intersection of the two streams entered the heated capillary inlet of a mass spectrometer. The mass spectrum was recorded as a function of the distance x between the mass spectrometer inlet and the droplet fusion center. Fused droplet trajectories were imaged with a high-speed camera, revealing that the droplet fusion occurred approximately within a 500-μm radius from the droplet fusion center and both the size and the speed of the fused droplets remained relatively constant as they traveled from the droplet fusion center to the mass spectrometer inlet. Evidence is presented that the reaction effectively stops upon entering the heated inlet of the mass spectrometer. Thus, the reaction time was proportional to x and could be measured and manipulated by controlling the distance x. Kinetic studies were carried out in fused water droplets for acid-induced unfolding of cytochrome c and hydrogen–deuterium exchange in bradykinin. The kinetics of the former revealed the slowing of the unfolding rates at the early stage of the reaction within 50 μs. The hydrogen–deuterium exchange revealed the existence of two distinct populations with fast and slow exchange rates. These studies demonstrated the power of this technique to detect reaction intermediates in fused liquid droplets with microsecond temporal resolution.
Journal information: Proceedings of the National Academy of Sciences
© 2015 Phys.org