Radiochemistry Annex: It's getting hot in there

Scientist Daniel Kaplan has found it challenging to study radionuclides in contaminated wetlands due to the radioactive hazard and the biogeochemical complexity of the subsurface soils. Fortunately, he's able to safely pursue his research at EMSL's Radiochemistry Annex.
"Very few facilities in the U.S. have capability to do radiological work, the highly specialized equipment and scientific expertise of the Radiochemistry Annex," says Kaplan, a senior fellow scientist at Savannah River National Laboratory. He's one of several scientists who are working with EMSL through a Special Science Call for Proposals to do high-impact research at the Annex.
Located across the street from the main EMSL facility, the Annex opened in early 2014. It offers 6,000 square feet of lab space designed to safely study radionuclide samples in terrestrial and subsurface ecosystems. It houses advanced spectroscopic and imaging technologies as well as a full suite of instruments for sample preparation and analysis in a contiguous space. Like all EMSL capabilities, those in the Annex are available to the scientific community at typically no cost for openly published research.
Uranium Sequestration
Jeffrey Catalano, an associate professor at Washington University in St. Louis, was one of the prospective users approved to utilize the Annex's capabilities during the Special Science Call. Working with him are Daniel Giammar and Lindsay Troyer, both with Washington University in St Louis. They are studying the use of phosphate to remediate subsurface uranium contamination with funding by the Department of Energy's Office of Biological and Environmental Research.
"One of BER's mission goals is to develop a robust scientific understanding of physical, chemical and biological processes of contaminated subsurface systems for effective decision making on environmental remediation and stewardship," says Catalano.
At sites around the world, including some DOE facilities, uranium has been released into the subsurface. Uranium has become dissolved in the groundwater and can migrate toward waterways. These sites are challenging to treat because uranium is spread over a large area deep below the ground. One possible remediation approach is with an in situ treatment method. This entails injecting a chemical into the contaminated area that makes uranium less soluble so that it does not migrate in groundwater, thus reducing the potential health hazard posed by the radionuclide.
Other researchers tested an in situ treatment method by injecting dissolved phosphate into the subsurface at the Hanford Site in southeastern Washington. The intent was for the phosphate to react with uranium to create uranium-phosphate solids, insoluble phases that would not migrate.
"It turns out the subsurface system is a bit more complicated than previously thought," says Catalano. "The initial tests didn't work as expected."
According to Catalano, the chemical reactions caused by adding phosphates to a subsurface system aren't well understood enough to be able to predict what will happen when this remediation method is used. His team's experimental investigation at the Annex is trying to understand when uranium-phosphate solids form, their composition and solubility, what other solids form and what other reactions occur.
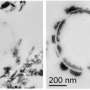
Catalano and his team have been working on this phosphate remediation project for three years, but they have only been utilizing EMSL's capabilities through the Special Science Call since September. As part of the proposal, they are using EMSL's fluorescence, cryogenic spectrometer to determine the chemical forms of uranium in the samples formed under different conditions. With the radiological X-ray photoelectron spectrometer, better known as XPS, they can study nucleation behavior on mineral surfaces to determine how much uranium and phosphate are on the mineral surfaces. They're also investigating field-site sediments, in particular the calcium-phosphate solids, under high resolution microscopy with the Annex's radiological focused ion beam scanning electron microscope, or FIB/SEM, and radiological transmission electron microscope, or TEM.
At this point in the study, most of the EMSL data is from the fluorescence, cryogenic spectrometer. They are finding uranium-phosphate solids only precipitate under a limited set of conditions. In most cases, uranium instead adsorbs to other solids, likely newly formed calcium phosphates, and can actually be easily remobilized when phosphate addition ends.
"Until we had the EMSL data, we didn't know the chemical reactions weren't forming dedicated uranium-phosphate solids," says Catalano. "And that's not what we expected."
Catalano anticipates receiving XPS data in mid-December, and the microscopy work will be done in the spring.
"We're just starting to get data from EMSL, but from what I've seen so far, the information from the Radiochemistry Annex will help us make better predictions of how effective remediation methods are over the longer term," says Catalano.
Hematite-technetium Interface
Nathalie Wall, an assistant professor of chemistry at Washington State University, is another scientist whose proposal to use the Annex was approved through the Special Science Call. She is supervising Larissa Gribat, a WSU graduate student, to study the chemistry of the hematite-technetium interface and its effect on technetium mobility. The Department of Defense is funding this study.
Technetium, or Tc, is a radioactive fission product often present in nuclear waste. It tends to be more mobile in the environment than other fission products.
"There is a lack of knowledge of the fundamental chemistry of Tc that would explain its mobility in complex systems, such as the environment," says Wall.
Tc in the environment has two oxidation states, Tc(VII) and Tc(IV). Generally, Tc(VII) is soluble and highly mobile in the environment, especially in water; and Tc(IV) is insoluble and quite immobile. It was thought the best way to remediate Tc(VII) was to add a reducing agent, microbes or a mineral to form Tc(IV) and immobilize it. Unfortunately, this remediation process only works in the laboratory, not in an environmental system. Tc(IV) can form soluble, mobile compounds with molecules in nuclear waste or the environment.
"The idea Tc in one state is very soluble compared to the other one isn't completely correct," says Wall. "Tc(IV) is less soluble than Tc(VII), but in an environmental system it's not insoluble; it's still slightly soluble. The problem is we don't know exactly how much, because there's a lack of thermo-dynamic data."
Wall and Gribat designed their Annex research to gather the needed data using a combination of radiochemistry, electrochemistry and inorganic chemistry. Previous research has shown iron, or Fe, effects on the oxidation state of Tc. Iron exists naturally in the environment as Fe(II) or Fe(III). They want to see if Fe(III)O2, or hematite, will re-oxidize Tc(IV) to Tc(VII) in an oxygen-free environment. Conversely, they want to put Tc(VII) in contact with an Fe(II) mineral and see if it reduces it to Tc(IV). They are using the Annex's XPS with attached environmental chamber to determine the oxidations state of the Tc samples.
"To understand the mobility of Tc, we need to understand the switch between the two Tc oxidation states – it's never been looked at in a systematic way," says Wall. "The reason we're doing this study is to see how an iron mineral affects the redox of Tc, because the redox of Tc influences and drives the mobility of Tc in the environment."
For Wall, a bonus of doing research at the Annex is its proximity to WSU's main campus in Pullman, Wash. She can cost-effectively send her students to Richland to gain the experience of working at a national scientific user facility with respected scientists. Gribat has made several trips to EMSL to oversee the project since it started two months ago. Wall says it has been an outstanding experience for Gribat.
Wall and Gribat's study aligns with BER's missions. Their findings will be helpful when remediating subsurface systems contaminated with Tc, including the Hanford Site and other DOE facilities. In addition, by knowing more about Tc chemistry, nuclear waste repositories can be designed to ensure Tc does not escape the boundary of the repository.
"This research will lead to a better understanding of Tc chemistry for better remediation and better containment of future nuclear waste. We'll have less environmental remediation to do in the future if we can fix the problem now," says Wall. "And by better understanding how Tc chemistry works in the environment, we can find a way to stop it. That will be a huge savings by not having to go back and remediate contaminated sites."
Uranium Redox Cycling
Kaplan is the principal investigator and Peter Jaffe, Princeton University, is his colleague on the wetland study in the Annex. Their BER-funded research explores the influence of iron and carbon on uranium redox cycling in wetlands with a focus on the plant root zone, or rhizosphere.
Kaplan and Jaffe are studying uranium contaminated wetlands on DOE's Savannah River Site. Over time, uranium radionuclides travel through the vadose zone – the area between the surface and the aquifer – and move through the aquifer and collect in a wetland or riparian zone until migrating into a waterway. In support of BER's missions, this research will help show how legacy radioactive waste is impacting the environment and will be used to help estimate any potential risk.
Wetlands consist of the rhizosphere and bulk soil, the area not penetrated by roots. A lot of geochemical reactions take place in wetlands due to the organic matter and microbial colonies. Their research at EMSL has discovered a difference in the iron chemistry between the rhizosphere and bulk soil. The rhizosphere has more iron nanoparticles and a greater percentage of organic matter, and the properties of the organic matter in the rhizosphere are also different than those in bulk soil.
"The rhizosphere accounts for a very small percentage of the total volume of these wetlands, but it seem to be accounting for a disproportionately large percent of uranium in that system," says Kaplan. "We're interested in finding the factors in the soils that have the greatest impact on chemistry – that's why we're focusing on the rhizosphere. Because of its scale – just millimeters – I've called on EMSL to help us to characterize the rhizosphere and do it with radioactive soils."
This Annex study will utilize a number of EMSL's spectrometry and microscopy capabilities, including the XPS, TEM, scanning probe microscope and electron microprobe. Kaplan and Jaffe chose to do their research at the Annex because of its unique instrumentation, ability to handle radioactive samples, and knowledgeable scientists who are experts at helping to interpret data and putting it into the appropriate context for their study.
Kaplan and Jaffe's Special Science Call research is a follow-up to a previous project using EMSL resources but not at the Annex. They used data from the earlier study to develop the Special Science Call proposal. While Kaplan and Jaffe's research at the Annex has only been underway for a few months, their research using EMSL resources is generating valuable information.
"Our work at EMSL has shown the chemical and biological differences between the rhizosphere and bulk soil – these findings are groundbreaking," says Kaplan. "None of these differences has been demonstrated before with uranium-contaminated sediments. We could have never done it without EMSL instrumentation and staff support."
Provided by Pacific Northwest National Laboratory