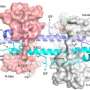
Tarantula toxin is used to report on electrical activity in live cells
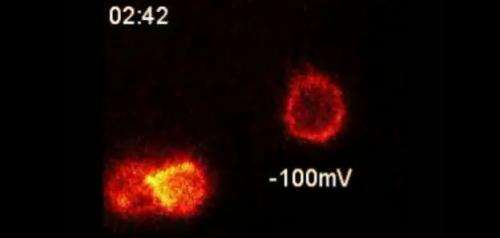
Crucial experiments to develop a novel probe of cellular electrical activity were conducted in the Neurobiology course at the Marine Biological Laboratory (MBL) in 2013. Today, that optical probe, which combines a tarantula toxin with a fluorescent compound, is introduced in a paper in the Proceedings of the National Academy of Sciences.
The lead authors of the paper are Drew C. Tilley of UC-Davis and the late Kenneth Eum, a Ph.D. candidate at UC-Davis and teaching assistant in the MBL Neurobiology course.
The probe takes advantage of the potent ability of tarantula toxin to bind to electrically active cells, such as neurons, while the cells are in a resting state. The team discovered that a trace amount of toxin combined with a fluorescent compound would bind to a specific subset of voltage-activated proteins (Kv2-type potassium ion channels) in live cells. The probe lights up cell surfaces with this ion channel, and the fluorescent signal dims when the channel is activated by electrical signals.
This is the first time that researchers have been able to visually observe these ion channels "turning on" without first genetically modifying them. All that is required is a means to detect probe location, suggesting that related probes could potentially one day be used to map neural activity in the human brain.
"This is a demonstration, a prototype probe. But the promise is that we could use it to measure the activity state of the electrical system in an organism that has not been genetically compromised," says senior author Jon Sack, an assistant professor in the departments of Physiology and Membrane Biology at UC-Davis. Sack is a faculty member in the MBL Neurobiology course.
Since the probe binds selectively to one of the many different kinds of ion channels, it can help scientists disentangle the function of those specific channels in neuronal signaling. This can, in turn, lead to the identification of drug targets for neurological diseases and disorders.
"We have an incredible diversity of ion channels, and even of voltage-activated ion channels. The real trouble has been determining which ones perform which roles. Which ones turn on and when in normal nervous system functioning? Which are involved in abnormal states or syndromes?" Sack says. "The dream is to be able to see what the different types of ion channels are doing and when, to understand what they contribute to physiology and pathophysiology."
These probes respond to movement of ion channel voltage sensors, and it is particularly fulfilling to have conducted some of this work at the MBL, Sack says. The first measurements of voltage sensor movement were conducted at the MBL in the early 1970s by Clay M. Armstrong and Francisco Bezanilla (Nature 242: 459-461, 1973). Armstrong and Bezanilla used electrophysiological methods to measure the movement of voltage sensors. The spider toxin probe create an optical signal when voltage sensors move, and no electrophysiology or genetic mutation of the channels is required.
More information: Tilley DC, Eum KS, Fletcher-Taylor S, Austin DC, Dupré C, Patrón LA, Garcia RL, Yarov-Yarovoy V, Cohen BE, and Sack JT (2014) Chemoselective tarantula toxins report voltage activation of wild-type ion channels in live cells. PNAS: www.pnas.org/cgi/doi/10.1073/pnas.1406876111
Journal information: Proceedings of the National Academy of Sciences , Nature
Provided by Marine Biological Laboratory