Organic tin in polymers increases their light absorption
Researchers of the Christian-Albrechts-University of Kiel (CAU), Germany, successfully integrated organic tin into semiconducting polymers (plastics) for the first time. Semiconducting polymers can be used, for example, for the absorption of sun light in solar cells. By incorporating organic tin into the plastic, light can be absorbed over a wide range of the solar spectrum. The new polymer is introduced by project leader Professor Anne Staubitz and the Ph. D. student Julian Linshöft in the renowned professional journal "Angewandte Chemie, International Edition."
Contrary to electric conductors such as metals, semiconductors are materials that conduct electricity only under certain circumstances, for example under irradiation with light. Because of this property semiconducting plastics (also called semiconducting polymers) are highly promising materials for the latest generation of solar cells – organic solar cells. Compared to the classical inorganic variants, their fabrication can be cheaper and they are very light weight materials, which can be advantageous for many applications, for example in the transport sector. "However, organic solar cells still do not achieve the same efficiencies as inorganic solar cells based on silicon so that there is a substantial need for research in this area", Anne Staubitz from the Otto Diels-Institute places her research area into context.
An important criterion of such semiconductors is how efficiently they absorb sun light in order to convert it into electricity. When sun light is converted into electricity, negatively charged electrons in the semiconductor are being lifted from one energy level into a higher energy level. This process leaves behind a positively charged "hole" in the lower energy level. Then the charges are percolating separately to the different electrical poles: A current can be observed. Sun light is able to initiate this process. The closer these energy levels are together, the more facile this process is: More photons can be absorbed and thus more solar energy can be used. Polymers, in which this gap ("band gap") between the energy levels is small, have a red, in rare cases even a purple colour.
One aim of synthetic organic semiconductor research is therefore to produce organic polymers with small energy gaps (or band gaps). However, the development of such strongly light absorbing, deeply coloured plastics is very difficult and therefore a very active area in current research. "With the new material from our laboratories, it is visible to the naked eye that we were successful in developing such plastics!" says Staubitz. The polymer is deep purple in solution and almost black when processed into a thin film.

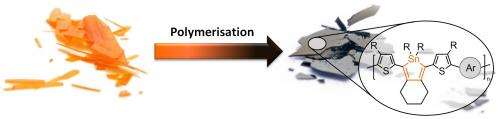
In order to achieve very small energy gaps, the scientists from Kiel used a new concept. They incorporated organic tin in the form of cyclic molecules ("stannoles") into the carbon-polymer backbone. Tin belongs to the same chemical group as carbon and is therefore similar in some of its properties. The electronic properties however between stannoles and the corresponding carbon congeners (cyclopentadienes) are very different. "Tin is not just an overweight carbon atom", Anne Staubitz explains. "It can lower the energetic levels in its organic compounds dramatically." But until now, nobody was able to use these special properties of tin in polymeric materials.
Joining these individual molecular building blocks (the monomers) together was a difficult task for the researchers: The monomers did not only contain the desired tin in the stannole-units themselves; organic tin was also present in the reactive coupling groups that were necessary for joining the monomers together to form the polymer. Only these groups were supposed to react, whereas the stannole rings should not be attacked. This was vital, because any undesired side reaction would lead to a significant shortening of the polymer chain, leading to a substantial deterioration of the polymer's quality. "This was a high risk project, because coupling reactions that can select between two different organic tin groups were not known in chemistry before", Staubitz says. Therefore, Ph. D. student Julian Linshöft did not just have to develop a selective, but a highly selective cross-coupling reaction. "The first difficulty was to find the correct reactivity patterns for the monomers", Linshöft recalls. "For this, there was no lead in the chemical literature so far."
The experiment was a success. The team was able to prepare the desired plastic using palladium as a reaction catalyst. The material can be processed easily into thin films, which are gleaming black and whose application in solar cells can now be tested. Linshöft, whose work was funded by a scholarship of the German Foundation for the Environment says: "Finally, we are able to prepare these new semiconducting plastics. Their full potential can be assessed in the near future."
-

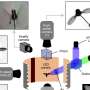
The polymer can be processed from solution to form a thin film. The technology, which is used here, is called spin coating. A solution is being dripped onto a fast rotating disc and the solvent is flung away. In the photo: Julian Linshöft is preparing a polymer film. Credit: Grace Suana -
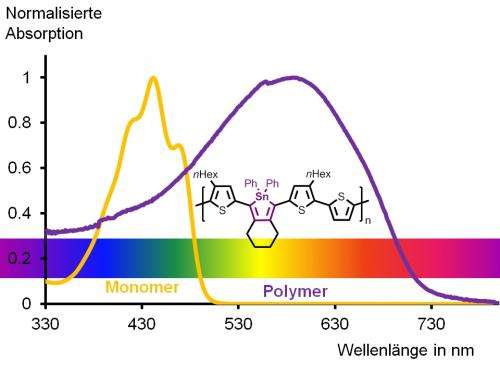
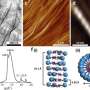
The absorption of the polymer extends well into the orange area of the spectrum, so that the eye can only see parts of the spectrum in the longer wavelength area. Therefore, the polymer appears optically purple in solution. The organic tin heterocycle, the stannole, is marked in purple in the chemical structure. Credit: Anne Staubitz
More information: Julian Linshoeft, Evan J. Baum, Andreas Hussain, Paul J. Gates, Christian Näther and Anne Staubitz. Highly Tin Selective Stille Coupling: Synthesis of a Polymer Containing a Stannole in the Main Chain. Angewandte Chemie, International Edition DOI: 10.1002/anie.201407377
Journal information: Angewandte Chemie International Edition
Provided by Kiel University