World's coolest molecules
It's official. Yale physicists have chilled the world's coolest molecules.
The tiny titans in question are bits of strontium monofluoride, dropped to 2.5 thousandths of a degree above absolute zero through a laser cooling and isolating process called magneto-optical trapping (MOT). They are the coldest molecules ever achieved through direct cooling, and they represent a physics milestone likely to prompt new research in areas ranging from quantum chemistry to tests of the most basic theories in particle physics.
"We can start studying chemical reactions that are happening at very near to absolute zero," said Dave DeMille, a Yale physics professor and principal investigator. "We have a chance to learn about fundamental chemical mechanisms."
The research is published this week in the journal Nature.
Magneto-optical trapping has become ubiquitous among atomic physicists in the past generation—but only at the single-atom level. The technology uses lasers to simultaneously cool particles and hold them in place. "Imagine having a shallow bowl with a little molasses in it," DeMille explained. "If you roll some balls into the bowl, they will slow down and accumulate at the bottom. For our experiment, the molecules are like the balls and the bowl with molasses is created via laser beams and magnetic fields."
Until now, the complicated vibrations and rotations of molecules proved too difficult for such trapping. The Yale team's unique approach drew inspiration from a relatively obscure, 1990s research paper that described MOT-type results in a situation where the usual cooling and trapping conditions were not met.
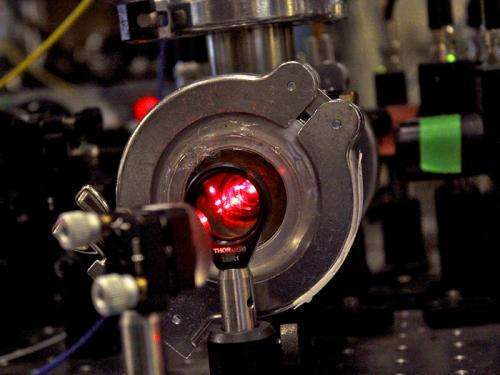
DeMille and his colleagues built their own apparatus in a basement lab. It is an elaborate, multi-level tangle of wires, computers, electrical components, tabletop mirrors, and a cryogenic refrigeration unit. The process uses a dozen lasers, each with a wavelength controlled to the ninth decimal point.
"If you wanted to put a picture of something high-tech in the dictionary, this is what it might look like," DeMille said. "It's deeply orderly, but with a bit of chaos."
It works this way: Pulses of strontium monofluoride (SrF) shoot out from a cryogenic chamber to form a beam of molecules, which is slowed by pushing on it with a laser. "It's like trying to slow down a bowling ball with ping pong balls," DeMille explained. "You have to do it fast and do it a lot of times." The slowed molecules enter a specially-shaped magnetic field, where opposing laser beams pass through the center of the field, along three perpendicular axes. This is where the molecules become trapped.
"Quantum mechanics allows us to both cool things down and apply force that leaves the molecules levitating in an almost perfect vacuum," DeMille said.
The Yale team chose SrF for its structural simplicity—it has effectively just one electron that orbits around the entire molecule. "We thought it would be best to start applying this technique with a simple diatomic molecule," DeMille said.
The discovery opens the door for further experimentation into everything from precision measurement and quantum simulation to ultracold chemistry and tests of the standard model of particle physics.
Publication details
Magneto-optical trapping of a diatomic molecule, Nature 512, 286–289 (21 August 2014) DOI: 10.1038/nature13634
Journal information: Nature
Provided by Yale University




















