New Nano3 microscope will allow high-resolution look inside cells

The University of California, San Diego's Nanofabrication Cleanroom Facility (Nano3) is the first institution to obtain a novel FEI Scios dual-beam microscope, with an adaptation for use at cryogenic temperatures. The new microscope will enable research among a highly diverse user base, ranging from materials science to structural and molecular biology.
As Nano3 Technical Director Bernd Fruhberger explains: "There is a tremendous interest in utilizing this instrument among faculty from multiple departments. The Departments of Nanoengineering, Materials and Aerospace Engineering, Electrical and Computer Engineering, Chemistry, Physics and Biology at UC San Diego all have projects in need of this tool, and have been actively involved in making the procurement of the tool a reality.
"The instrument provides state-of-the-art capabilities for cross-sectioning, preparation of sections for Transmission Electron Microscopy and more," he adds, "but what truly differentiates it is the novel cryo-capability, which will make it possible for cell biologists to see the structures of biological cells in higher resolution to better understand how cells function at a molecular level. This could possibly pave the way for new treatments and drug discovery."
Elizabeth Villa, a new assistant professor in the Department of Chemistry & Biochemistry at UC San Diego, along with her colleagues at Germany's Max Planck Institute of Biochemistry, adapted a focused-ion-beam microscope for biological applications during her postdoctoral studies. The design was adopted by the Dutch company FEI into a first-of-a-kind prototype that Villa will further develop in UC San Diego in collaboration with the company.
Villa notes that UC San Diego has an established academic tradition in the area of molecular imaging – most notably reflected in the work of biochemist Roger Tsien. Tsien won the 2008 Nobel Prize in Chemistry for the discovery and development of the green fluorescent protein, which revolutionized the fields of cell biology and neurobiology by allowing scientists to peer inside living cells and watch their behavior in real time.
"What I'm doing is similar," explains Villa, "only I'm using electron microscopy, which gives us higher-resolution images. The idea behind our method is to bring together people who do structural biology with people who do cell biology by using a new tool that will allow us to see the structures of the cells, at high resolution, and better understand what molecules are doing."
To explain the difference between light microscopy (which made Tsien's work possible) and her work in electron microscopy, Villa invokes a metaphor.
"Light microscopy is like giving lanterns to a bunch of people in a city. You can see where those people are, but you can't see what's going on around them. With electron microscopy, you can see the people with lanterns (a cell's molecules) and you can also see the walls and buildings of the city (the cell's structure)."
But electron microscopy has its downside. Traditionally, to be visible, cells must be prepared beforehand by drying and staining them with what Villa equates to a "thick layer of paint." However, most cells are too thick to be studied this way, and that's what makes the Scios tool a game changer: It allows Villa to bypass the stain and nanomachine the cells to reduce them to the thickness required for electron microscopy – around a few tenths of a micron – without creating any sample distortions and while maintaining cryogenic temperatures (generally the temperature of liquid nitrogen).
Discover the latest in science, tech, and space with over 100,000 subscribers who rely on Phys.org for daily insights. Sign up for our free newsletter and get updates on breakthroughs, innovations, and research that matter—daily or weekly.
Adds Villa: "There are people on campus—like Neuroscience Professor Mark Ellisman—who do a magnificent job designing and using these types of stains, but when the goal is getting a high-resolution image of the cells where the question involves determining structural details, you want to avoid having this extra layer on top of them. It would be like having a layer of paint over your face and then trying to count how many eyelashes you have. You'd be out of business."
Villa compares the process of studying cells (typically eukaryotic cells, in her case) at cryogenic temperatures to 'flash freezing' the cellular 'city' in her previous metaphor.
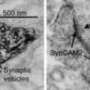
"Everything in the cell freezes in the position it was in so we can get a better look," she says. "One thing I've been studying is something known as the nuclear pore complex, which is the gatekeeper of the nucleus. It keeps the DNA inside the nucleus and away from the other parts of the cell. If we were to take it out of the cell entirely to study it, it wouldn't make a whole lot of sense, which is why we have to freeze it in place.
"With cryo-electron tomography techniques, we can create 3D pictures of the cells called tomograms," she continues. "What I do is exactly equivalent to a CT (computed tomography) scan, except the cells are a million times smaller. We can take those 3D images and look at them in the (Qualcomm Institute's) StarCAVE or NexCAVE enlarged and in color, and get an even better sense for what's going on."
Villa adds that another benefit of cryo-electron microscopy is the ability to infer cellular dynamics over time, "or what we call in physics 'ergodicity.' I can look at 3,000 nuclear pores frozen at different times to infer the cellular dynamics, classify all of this information and then make predictions. We can then do a light microscopy experiment in vivo (with a live cell) and correlate what we see with the previous data we've gathered."
Villa points out that by using the Scios dual beam for nanomachining biological material she's in a sense, "hijacking a tool that materials scientists use all the time in the nanofabrication of materials."
The Scios microscope will also facilitate planned UC San Diego-led research in neurodegenerative diseases, says Villa, as well as research pertaining to cancer and heart disease.
"Many kind of perturbations or phenotypes that come from disease or recovery from disease are going to be able to be examined using the Scios microscope," she notes. "It's important to note this is a first step and there remains much work to be done, but it places us in a really exciting place where we aim to look at molecular structures in their natural context: from the cell to the organism level."
Provided by University of California - San Diego