Protective hinge process enables insulin to bind to cells
Since its landmark discovery in 1922, insulin has improved the health and extended the lives of more than 500 million people worldwide with diabetes mellitus. Yet the question of how this key hormone binds to its target cells in the body's organs has posed an enduring scientific mystery. A global team of researchers from Cleveland, Australia, Chicago, India and Oregon has made a discovery about insulin and its structure that promises to enable design of new insulin products that will do a better job of regulating the metabolism of patients with diabetes.
The scientists, co-led by Michael A. Weiss, MD, PhD (Case Western Reserve School of Medicine, Cleveland) and Michael C. Lawrence, PhD (Walter and Eliza Hall Institute and the University of Melbourne, Australia), deciphered how the insulin molecule exploits a "protective hinge" to engage its primary binding site within the insulin receptor. The results of the team's interdisciplinary research appeared the first week of August in an online edition of PNAS (Proceedings of the National Academy of Sciences). Solving this problem required integration of synthetic, biochemical, biological, spectroscopic and crystallographic approaches.
"We discovered an essential mechanism for how insulin binds to target cells and thereby triggers an extraordinary cascade of biological signals," said Weiss, chairman of the Department of Biochemistry and Distinguished Research Professor at the Case Western Reserve School of Medicine. "Such molecular signaling, central to how we utilize and store fuels derived from our meals, has attracted international scientific study ever since the landmark 1969 elucidation of the storage structure of insulin by the late Nobel Laureate Dorothy C. Hodgkin in England."
In this investigation, Weiss, Lawrence and their colleagues discovered a protective hinge within insulin that, when closed, ensures that the hormone safely remains in a storage form until it is appropriate to open—a structural transformation that allows docking to the surfaces of target cells of muscle, liver, fat and other tissues. Such docking is the first step in metabolic signaling, which, for example, enables the target cells to take in glucose (the sugar building block) and thereby avoid a build-up of glucose in the blood stream (hyperglycemia), a cardinal feature of diabetes mellitus.
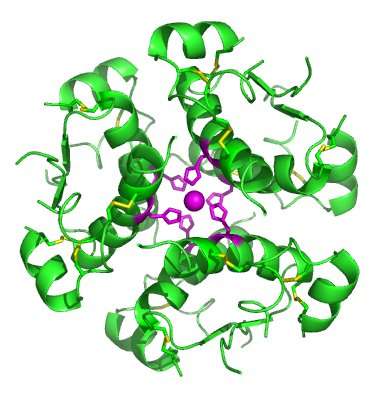
Investigators uncovered the protective hinge by observing the intricate structural features as visualized in crystal structures in whose building blocks a single molecule of insulin is bound to fragments of the insulin receptor. Past studies, including the classical crystallographic studies by insulin structure pioneer Hodgkin, focus on groups of six insulin molecules (hexamers) in the absence of the receptor. This closed form of insulin is pertinent to how it is stored in the body or prepared in a pharmaceutical formulation. The hexamers contain three pairs (dimers) of insulin molecules. Each dimer contains a crossing point of eight aromatic rings, four from each insulin molecule. (Aromatic rings are closed-ring structures formed by carbon atoms within the molecule.) In the new pictures of the open and active form of the hormone, these aromatic rings dock into pockets of the cellular receptor. Insulin thus opens a hinge to expose its functional surface.
"We believe that the closed form of insulin evolved to permit its efficient production and safe storage within the pancreas," said Weiss. "Yet variant forms of insulin stabilized in this state have no biological activity."
These groundbreaking findings have led investigators to the next stage of research—how to translate this discovery to make safer and more effective insulin products for patients. The ultimate goal is to develop new molecular forms of insulin that will ensure that the protective hinge opens within the insulin only when it should. Possible versions of newer, more effective insulin modalities are impressive: ultra-fast acting insulin formulations for "smart pumps," a strategic goal of the National Institutes of Health and the Juvenile Diabetes Research Foundation (JDRF); ultra-stable modes of insulin, which would benefit patients in the developing world with limited access to refrigeration; and even "smart" insulin molecules themselves, which stop working when the concentration of glucose in the blood goes below normal. Improvements in insulin safety and effectiveness promise to reduce the risk of long-term health consequences from diabetes such as kidney failure, blindness and foot amputations.
"We have addressed a real-world problem that has been part of a more than 40-year exploration for how insulin is made in the body, how it folds in the specialized beta-cells of the pancreas until it is ready for use, how it binds to a receptor in the cell and how the insulin degrades," Weiss said. "Promising new molecular designs for insulin are under study at Case Western Reserve and around the world that address all aspects of insulin structure, including optimization of the protective hinge."
Characterizing the insulin molecule has taken decades of research, and continues to this day: First, researchers sought to understand what insulin looks like when it is stored in the beta cell of the pancreas. Second, they need to show what insulin looks like when it is bound to the insulin-accepting receptor on the cell. Third, they want to illustrate how the receptor changes its shape in response to insulin binding to transmit a signal across the cell.
"Substantial progress toward the second milestone has been made by the present international collaborative team," Weiss said. "It is extraordinarily rare, and it is a privilege, to be part of such a team. We have sharper pictures now, and for the first time, we can visualize the part of the insulin molecule that is changing its shape and so looking different than in the landmark Hodgkin structure of 1969."
More information: Protective hinge in insulin opens to enable its receptor engagement, PNAS, www.pnas.org/cgi/doi/10.1073/pnas.1412897111
Journal information: Proceedings of the National Academy of Sciences
Provided by Case Western Reserve University