Chemists seek state-of-the-art lithium-sulfur batteries
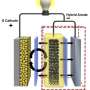
When can we expect to drive the length of Germany in an electric car without having to top up the battery? Chemists at the NIM Cluster at LMU and at the University of Waterloo in Ontario, Canada, have now synthesized a new material that could show the way forward to state-of-the-art lithium-sulfur batteries.
Whether or not the future of automotive traffic belongs to the softly purring electric car depends largely on the development of its batteries. The industry is currently placing most of its hopes in lithium-sulfur batteries, which have a very high storage capacity. Moreover, thanks to the inclusion of sulfur atoms, they are cheaper to make and less toxic than conventional lithium-ion power packs.
However, the lithium-sulfur battery still presents several major challenges that need to be resolved until it can be integrated into cars. For example, both the rate and the number of possible charge-discharge cycles need to be increased before the lithium-sulfur battery can become a realistic alternative to lithium-ion batteries.
Lots of pores for sulfur
The chemists Professor Thomas Bein (LMU), Coordinator of the Energy Conversion Division of the Nanosystems Initiative Munich, Professor Linda Nazar (University of Waterloo, Waterloo Institute of Nanotechology) and their colleagues have now succeeded in producing a novel type of nanofiber, whose highly ordered and porous structure gives it an extraordinarily high surface-to-volume ratio. Thus, a sample of the new material the size of a sugar cube presents a surface area equivalent to that of more than seven tennis courts.
"The high surface-to-volume ratio, and high pore volume is important because it allows sulfur to bind to the electrode in a finely divided manner, with relatively high loading. Together with its easy accessibility, this enhances the efficiency of the electrochemical processes that occur in the course of charge-discharge cycles. And the rates of the key reactions at the sulfur electrode-electrolyte interface, which involve both electrons and ions, are highly dependent on the total surface area available," as Benjamin Mandlmeier, a postdoc in Bein's Institute and a first co-author on the new study, explains.
The secret recipe
A novel recipe and a cleverly designed mode of synthesis are the key factors that determine the properties of the new materials. To synthesize the carbon fibers, the chemists first prepare a porous, tubular silica template, starting from commercially available, but non-porous fibers. This template is then filled with a special mixture of carbon, silicon dioxide and surfactants, which is then heated at 900°C. Finally the template and the SiO2 are removed by an etching process. During the procedure, the carbon nanotubes – and thus the pore size – shrink to a lesser extent than they would in the absence of the confining template, and the fibers themselves are correspondingly more stable.
"Nanostructured materials have great potential for the efficient conversion and storage of electrical energy," says Thomas Bein. "We in the NIM Cluster will continue to collaborate closely with our colleagues in the Bavarian SolTech Network in order to explore and exploit the properties of such structures and their practical applications."
More information: "Bimodal Mesoporous Carbon Nanofibers with High Porosity: Freestanding and Embedded in Membranes for Lithium–Sulfur Batteries." Guang He, Benjamin Mandlmeier, Jörg Schuster, Linda F. Nazar, and Thomas Bein. Chemistry of Materials Article ASAP. DOI: 10.1021/cm403740r
Journal information: Chemistry of Materials
Provided by Ludwig Maximilian University of Munich