New super waterproof surfaces cause water to bounce like a ball

(Phys.org) —In a basement lab on BYU's campus, mechanical engineering professor Julie Crockett analyzes water as it bounces like a ball and rolls down a ramp.
This phenomenon occurs because Crockett and her colleague Dan Maynes have created a sloped channel that is super-hydrophobic, or a surface that is extremely difficult to wet. In layman's terms, it's the most extreme form of water proof.
Engineers like Crockett and Maynes have spent decades studying super-hydrophobic surfaces because of the plethora of real-life applications. And while some of this research has resulted in commercial products that keep shoes dry or prevent oil from building up on bolts, the duo of BYU professors are uncovering characteristics aimed at large-scale solutions for society.
Their recent study on the subject, published in academic journal Physics of Fluids, finds surfaces with a pattern of microscopic ridges or posts, combined with a hydrophobic coating, produces an even higher level of water resistance—depending on how the water hits the surface.
"Our research is geared toward helping to create the ideal super-hydrophobic surface," Crockett said. "By characterizing the specific properties of these different surfaces, we can better pinpoint which types of surfaces are most advantageous for each application."
Their work is critical because the growing list of applications for super-hydrophobic surfaces is extremely diverse:
- Solar panels that don't get dirty or can self-clean when water rolls off of them
- Showers, tubs or toilets you don't want hard water spots to mark
- Bio-medical devices, such as the interior of tubes or syringes that deliver fluids to patients
- Hulls of ships, exterior of torpedoes or submarines
- Airplane wings that will resist wingtip icing in cold humid conditions
But where Crockett and Maynes' research is really headed is toward cleaner and more efficient energy generation. Nearly every power plant across the country creates energy by burning coal or natural gas to create steam that expands and rotates a turbine. Once that has happened, the steam needs to be condensed back into a liquid state to be cycled back through.
If power plant condensers can be built with optimal super-hydrophobic surfaces, that process can be sped up in significant ways, saving time and lowering costs to generate power.
"If you have these surfaces, the fluid isn't attracted to the condenser wall, and as soon as the steam starts condensing to a liquid, it just rolls right off," Crockett said. "And so you can very, very quickly and efficiently condense a lot of gas."

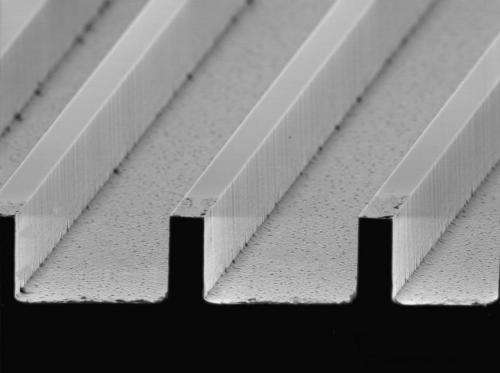
The super-hydrophobic surfaces the researchers are testing in the lab fall into one of two categories: surfaces with micro posts or surfaces with ribs and cavities one tenth the size of a human hair. (See images of each to the right.)
To create these micro-structured surfaces, the professors use a process similar to photo film development that etches patterns onto CD-sized wafers. The researchers then add a thin water-resistant film to the surfaces, such as Teflon, and use ultra-high-speed cameras to document the way water interacts when dropped, jetted or boiled on them.
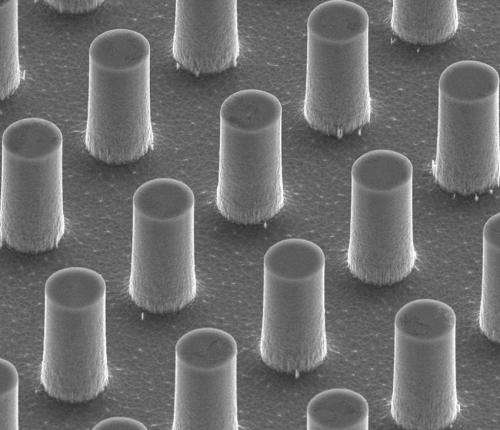
They're finding slight alterations in the width of the ribs and cavities, or the angles of the rib walls are significantly changing the water responses. All of this examination is providing a clearer picture of why super-hydrophobic surfaces do what they do.
"People know about these surfaces, but why they cause droplets or jets to behave the way they do is not particularly well known," Crockett said. "If you don't know why the phenomena are occurring, it may or may not actually be beneficial to you."
Journal information: Physics of Fluids
Provided by Brigham Young University



















