A key facilitator of mRNA editing uncovered
Molecular biologists from Indiana University are part of a team that has identified a protein that regulates the information present in a large number of messenger ribonucleic acid molecules that are important for carrying genetic information from DNA to protein synthesis.
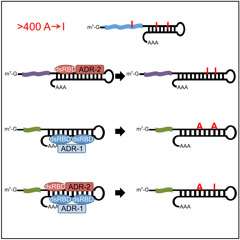
The new work, published today in Cell Reports, finds that the protein ADR-1 binds to messenger ribonucleic acid, or mRNA, and then enhances RNA editing, a process that allows a gene to be present as multiple mRNAs that can then each affect gene expression differently.
Organisms ranging from sea anemone to humans utilize RNA editing to express different mRNAs at various times in development. Decreased mRNA editing has been reported in patients with neuropathological diseases like epilepsy, schizophrenia, amyotrophic lateral sclerosis and several types of cancer, including glioblastomas (brain tumors).
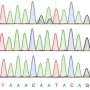
Using the model organism, Caenorhabditis elegans, the researchers identified over 400 new mRNA editing sites—the majority regulated by ADR-1—and declared the protein the first global regulator of RNA editing.
"What we've determined is that this protein's ability to alter editing of mRNAs is not specific to just a few genes, but instead, its ability to bind to mRNAs is required for proper RNA editing of most mRNAs," said Michael C. Washburn, a graduate student in the IU College of Arts and Sciences' Department of Biology and first author on the paper with Boyko Kakaradov of the University of California, San Diego.
Working in the laboratory of Heather A. Hundley, corresponding author on the paper and an assistant professor of biochemistry and molecular biology in the IU School of Medicine's Medical Sciences Program at Bloomington, Washburn and undergraduate Medical Sciences program student Emily Wheeler collaborated with the team from UCSD to show that the region of ADR-1 protein that binds to target mRNAs in C. elegans is also required for regulating editing. This region is present in many human proteins, and a protein similar to ADR-1 is specifically expressed in human neurons.
"So it is likely that a similar mechanism exists to regulate editing in humans," Hundley said. "Further work in our lab will be aimed at understanding the detailed mechanism of how these proteins regulate editing, in turn providing an inroad to developing therapeutics that modulate editing for the treatment of human diseases."
C. elegans is a microscopic worm that like humans highly expresses a family of proteins in the nervous system called ADARs—adenosine deaminases that act on RNA—a family that includes ADR-1.
ADARs change specific nucleotides (molecular building blocks for DNA and RNA) in RNA, in a process called adenosine-to-inosine editing, or A-to-I editing, that diversifies genetic information to specify different amino acids, splice sites and structures. Scientists currently estimate there are between 400,000 and 1 million A-to-I editing events in noncoding regions of the human transcriptome.
While newly synthesized RNA encodes the exact information found in DNA, it's when later RNA editing occurs that RNA gets altered, a change most often catalyzed by ADARs.
"One thing we also know is that ADAR protein levels are not altered in disease, implying that other mechanisms are also at work regulating ADAR-mediated RNA editing," Hundley said. "By identifying this major regulator of noncoding editing in C. elegans we can now focus on dissecting the regulatory mechanism and determining the conservation of this regulatory protein in human cells."
Journal information: Cell Reports
Provided by Indiana University