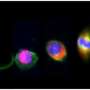
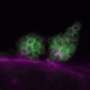
A paradigm-shifting step in stem cell research
(Phys.org) —A team of engineers at the University of Wisconsin-Madison has created a process that may revolutionize stem cell research. The process, outlined in a paper published in Stem Cells on December 19, 2013, will improve the state of the art in the creation of synthetic neural stem cells for use in central nervous system research.
Human pluripotent stem cells have been used to reproduce nervous-system cells for use in the study and treatment of spinal cord injuries and of diseases such as Parkinson's and Huntington's. Currently, most stem cells used in research have been cultured on mouse embryonic fibroblasts (MEFs), which require a high level of expertise to prepare. The expertise required has made scalability a problem, as there can be slight differences in the cells used from laboratory to laboratory, and the cells maintained on MEFs are also undesirable for clinical applications.
Removing the high level of required skill—and thereby increasing the translatability of stem cell technology—is one of the main reasons why Randolph Ashton, a UW-Madison assistant professor of biomedical engineering and co-author of the paper, wanted to create a new protocol.
Rather than culturing stem cells on MEFs, the new process uses two simple chemical cocktails to accomplish the same task. The first mixture, developed by John D. MacArthur Professor of Medicine James Thomson in the Morgridge Institute for Research, is used to maintain the stem cells in the absence of MEFs. The second cocktail allows researchers to push the stem cells toward a neural fate with very high efficiency.
These chemical mixtures help to ensure the consistency of the entire process and give researchers a better understanding of what is driving the differentiation of the cells. "Once you remove some of the confounding factors, you have better control and more freedom and flexibility in terms of pushing the neural stem cells into what you want them to become," says Ashton.
Streamlining the process also removes some of the ambiguities that were inserted with MEFs. And Ashton hopes the straightforward protocol will enable other labs to engage in more complex tissue engineering. "Ours is the simplest, fastest and most efficient way to generate these types of cells," he says.
Ethan Lippmann, a postdoctoral fellow at the Wisconsin Institute for Discovery and co-author on the paper, says the major impact of this new process on other labs will be two-fold. "It's incredibly easy and simplified, and you can buy everything 'off the shelf,' so to speak," he says. "This should allow other researchers who are not stem cell experts to adapt this protocol to their own labs. We also want people to look at the things we do, as we generate more specialized neural cell types using this protocol, and feel comfortable that they can be translated to a clinic."
Journal information: Stem Cells
Provided by University of Wisconsin-Madison