Biochemistry: New technique to study interaction of metal ions in a liquid

In August, the members of an ISOLDE project called LOI88 successfully employed a new technique to study the interaction of metal ions in a liquid. It's the first time that specific ions have been studied in a liquid medium - a technical achievement that opens promising doors for biochemistry.
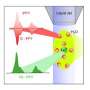
"More than half of the proteins in the human body contain metal ions such as magnesium, zinc and copper," explains Monika Stachura, a biophysicist at the University of Copenhagen and the LOI88 project leader. "We know that these elements are crucial to a protein's structure and function but their behaviour and interactions are not known in detail." Detecting these ions directly in a body-like environment is problematic as their closed atomic shells make them invisible to most spectroscopic techniques. However, using the beta-Nuclear Magnetic Resonance (β-NMR) technique in combination with the COLLAPS beamline the LOI88 team succeeded, for the first time, in recording a signal from metal ions in a body-like liquid environment. This also proves that basic nuclear physics research and techniques can lead to novel applications.
To obtain these excellent results, the team first had to meet a challenge: to find a way to introduce "easily visible" metal ions into a liquid, in order to then "see" their signal. And by "visible", ISOLDE physicists of course mean "radioactive". Their choice: radioactive magnesium 31 ions (31Mg++). The technique: β-NMR. The setup: complicated…
"First of all, we needed a Mg31 ion beam from ISOLDE," says Magdalena Kowalska, a β-NMR physicist participating in the project and the ISOLDE physics coordinator. "As we are using the NMR technique, we have to polarize the spins of these ions, which is done using laser light from the ISOLDE-COLLAPS set-up. The polarized ions are then caught by a drop of the liquid." Sounds easy? Not if you consider that the beam has to stay in a vacuum, but the liquid cannot. "When a liquid solution is placed in a vacuum it first boils and then freezes, making it impossible to perform the experiment," explains Alexander Gottberg, an ISOLDE target physicist from CSIC, Madrid, who designed the experimental set-up. "To overcome the problem, we had to introduce a pressure difference between the weak vacuum around the liquid target and the high vacuum in the beamline. The most challenging part of this design was that the differential pumping system, which was used for this purpose, had to be hosted on just a few centimeters."
Mg31 has a half-life of only 230 ms so, in less than a second, physicists can observe it decaying in the drop of liquid. And it's precisely this decay that gives the long-sought information. "By decaying, Mg31 emits beta particles," explains Magdalena. "As we polarized the Mg31 ions, this emission of beta particles isn't the same in all directions: we call this an 'anisotropy'." In simple words, scientists detect a different number of beta particles on "the left" detector than on "the right" one.
But what does this anisotropy mean? "From our theoretical models, we can deduce the interactions of the metal ions in the liquid by looking at the NMR radiofrequency that cancels the anisotropy," explains Alexander.
"By proving the feasibility of the technique, we have opened new doors for biochemistry," concludes Monika. "We are now preparing the next steps: injecting macromolecules and later proteins into the liquid to see how metal ions interact with them." The three experts confided that they were "extremely excited". No kidding!
Provided by CERN