June 8, 2012 feature
Wires turn salt water into freshwater

(Phys.org) -- As a rising global population and increasing standard of living drive demand for freshwater, many researchers are developing new techniques to desalinate salt water. Among them is a team of scientists from The Netherlands, who have shown how to transform brackish (moderately salty) water into potable freshwater using just a pair of wires and a small voltage that can be generated by a small solar cell. The simple technique has the potential to be more energy-efficient than other techniques because of the minimal amount of mixing between the treated and untreated water.
The researchers, led by Maarten Biesheuvel from Wageningen University in Wageningen, The Netherlands, and Wetsus, Centre of Excellence for Sustainable Water Technology in Leeuwarden, The Netherlands, have published their study on water desalination with wires in a recent issue of The Journal of Physical Chemistry Letters.
As the researchers explain in their study, there are two main ways to desalinate salt water. One way is to remove pure water molecules from the salt water, as done in distillation and reverse osmosis, particularly for water with a high salt concentration. The opposite approach is to remove the salt ions from the salt water to obtain freshwater, which is done in deionization and desalination techniques using, among other things, batteries and microbial cells.
Here, the scientists used the second approach, in which they removed positively charged sodium ions and negatively charged chlorine ions from brackish water to produce freshwater. To do this, they designed a device consisting of two thin graphite rods or wires, which are inexpensive and highly conductive. Then they coated the outer surface of the wires with a porous carbon electrode layer so that one wire could act as a cathode and one as an anode. The wires were clamped a small distance apart in a plastic holder, with each wire squeezed against a copper strip.
To activate the electrodes, the researchers dipped seven sets of wire pairs into a container of brackish water and ran electrical wires from the copper strips to an external power source. Upon applying a small voltage difference (1-2 volts) between the two graphite wires of each wire pair, one wire became the cathode and adsorbed the positively charged sodium cations, while the other wire became the anode and adsorbed the negatively charged chlorine anions from the salty water.
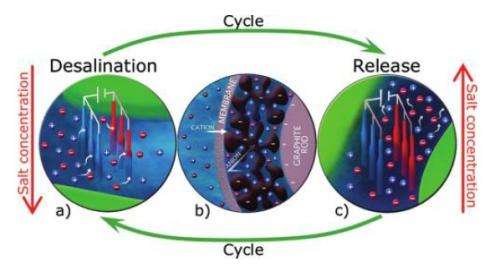
The ions are temporarily stored inside the nanopores of the carbon electrode coating until the wire pair is manually lifted from the once-treated solution and dipped into another container of waste water, or brine. Then the researchers removed the voltage, which caused the electrodes to release the stored ions into the waste water, increasing its salinity. By repeating this cycle eight times, the researchers measured that the salt concentration of the original brackish water, 20 mM (millimolars), is reduced to about 7 mM. Potable water is considered to have a salinity of less than roughly 15 mM. As Biesheuvel explained, this improvement could be useful for applications involving the treatment of moderately salty water.
“The new technique is not so suitable for extremely salty waters, as it is based on removing the salt, and making the remaining water less salty,” Biesheuvel told Phys.org, explaining that distillation and reverse osmosis are still superior for desalinating seawater (500 mM salinity and higher). “The new technique is more suitable, for example, for groundwater, or for water for consumer applications that needs to be treated to remove so-called ‘hardness ions’ and make it less saline. These water streams are less saline to start with, say 100 mM or 30 mM. Or this new approach can be of use to treat water in industry to remove ions (salts) that slowly accumulate in the process. In this way there is no need anymore to take in freshwater and/or to dump used water (at high financial penalty).”
Discover the latest in science, tech, and space with over 100,000 subscribers who rely on Phys.org for daily insights. Sign up for our free newsletter and get updates on breakthroughs, innovations, and research that matter—daily or weekly.
One of the biggest advantages of the technique is that it avoids inadvertently mixing the brine with the water being treated during the process, which limits the efficiency of other deionization techniques. By using a handheld wire-based device and producing freshwater in a continuous stream, the researchers could split the two types of water in separate containers to avoid mixing. Only a minimal amount of brine, about 0.26 mL per electrode, is transferred between containers, which does limit the degree of desalination but to a lesser extent than other techniques. Another advantage of the new technique is that it has the potential to be less expensive than other desalination methods.
“This technique can be made very inexpensive, just carbon rods or wires to conduct the electrons, onto which you can simply ‘paint’ the activated carbon slurry, which becomes the porous carbon electrode,” Biesheuvel said. “Because of its simplicity and low cost, it might out-compete state-of-the-art technologies for certain applications, and may also have advantages over the technology called capacitive deionization (CDI or cap-DI), which is beyond the development stage and commercially available. Also, the voltage required is low, just 1.2 V for instance, and DC, perfectly compatible with solar panels. Thus it can be used at off-grid or remote locations.”
In addition, Biesheuvel explained that the wire pairs can be used repeatedly without degradation, which could give the device a long lifetime.
“In capacitive techniques where the porous carbon electrodes are used to capture ions and release them again (in the so-called ‘electrical double layers,’ or EDLs, formed in the very small pores inside the carbon), it is well-known that the cycle can be used for thousands or tens of thousands of times (until the experimenter gets tired) without any appreciable decay,” he said. “For the wires we only went up to six times repeat and found, as expected, no changes. This is in contrast to battery-style techniques, either for energy storage or desalination, where one would expect to lose performance (like rechargeable batteries, which can only be charged, say, 100 times successfully). That is because in those techniques there is real chemistry going on, phase changes, change of the micromorphology of the anode/cathode materials. Here, in the wire desalination technology, nothing of that kind, the EDL is a purely physical phenomenon where ions are stored close to the charged carbon in the nanopores under the action of the applied voltage, and later released again.”
The researchers also found that the efficiency could be improved by adding a second membrane coating to the electrodes. For instance, a cationic membrane on the cathode wire has a high selectivity toward sodium cations while blocking the desorption of chlorine anions from within the electrode region. As a result, cationic (and, on the anode wire, anionic) membranes could enable the electrodes to adsorb and remove more ions than before.
In the future, the researchers plan to perform additional experiments using the cationic and anionic membranes. They predict that these improvements could increase the desalination factor from 3 to 4 after eight cycles, with 80% of the water being recovered (i.e., 20% of the original water becomes brine). The researchers also want to use the technique to treat large volumes of water, which they say could be done by using many wire pairs in parallel to accelerate the desalination process.
“This research continues by scaling up the technology (testing larger arrays of wires), packing them more closely, and trying our hand on automation to have the rods lifted automatically from one water stream into another,” Biesheuvel said. “We also want to test ‘real’ ground/surface waters, not only artificial simple salt mixtures as tested now.”
More information: S. Porada, et al. “Water Desalination with Wires.” The Journal of Physical Chemistry Letters. DOI: 10.1021/jz3005514
Journal information: Journal of Physical Chemistry Letters
Copyright 2012 Phys.org
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.