Nanotechnology promises better catalytic converter

(PhysOrg.com) -- Control over material properties would reduce the amount of platinum needed.
The toxic byproducts made when a car’s engine burns fuel are funneled into the catalytic converter, where chemical reactions turn them into much less toxic substances like water and carbon dioxide.
The catalyst that lowers the activation energy of these chemical reactions so that they can take place at reasonable temperatures and speeds is usually platinum, one of the world’s rarest and most precious metals. Because platinum is so expensive, automakers want to use as little as possible, and to use it as effectively as possible.
To maximize its specific surface area (surface area per unit mass) and therefore its chemical activity, manufacturers coat a ceramic support with small particles of platinum. But when the converter gets hot, the platinum aggregates, forming large clumps that cannot carry out the detoxification reactions as effectively. To compensate for the loss of efficiency, converters must contain more platinum, a scarce metal badly neede for other clean-energy applications, such as fuel cells.
A model catalytic system, described online this week of the German Chemical Society's Journal Angewantde Chemie International Edition, prevents the platinum from aggregating, so that less is needed for each converter.
The system was devised by a team of scientists including Younan Xia, PhD, the James M. McKelvey Professor of Biomedical Engineering in the School of Engineering and Applied Science at Washington University in St. Louis. The team also includes Charles T. Camvell, Phd, the Lloyd E. and Florence M. West Professor of Chemistry at the University of Washington in Seattle and Paul T. Fanson, PhD, a chemist at ToyotaToyota Motor Engineering & Manufacturing North America in Ann Arbor.
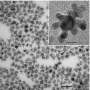
The key development is to coat platinum nanoparticles with a porous silica layer. Because of its weak interaction with the platinum, the silica coating provides an energy barrier that holds the platinum in place even at very high temperatures, preventing aggregation and maintaining catalytic activity.
The first step in making the new system is to load titanium dioxide nanofibers with platinum nanoparticles. This support makes the platinum catalyst more active by providing additional electrons for some of the detoxifying reactions. The loaded fibers are then coated with silica containing an organic pore-generating agent, which was then removed by heating to 350 degrees C to create porous sheaths.
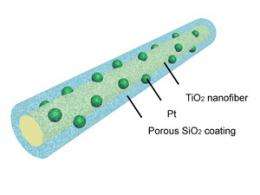
“It’s very tricky to make this kind of coating thin enough and porous enough so you don’t really affect the activity of the platinum catalyst. So that’s a major development,” Xia says.
Experiments then showed that the silica-coated platinum maintained its catalytic ability at much higher temperatures than uncoated platinum, which began to aggregate at temperatures as low as 350 degrees C.
Yunqian Dai, the lead author on the paper and a visiting graduate student from China, says this development “will greatly improve the thermostability” of platinum catalysts, although it is not yet clear if whether it will lead to new catalytic converter designs.
“It looks like we can run these up to 750 degrees without any significant agglomeration,” says Xia. “The typical temperature for a catalytic converter is about 550, so in that sense, it should be able to last for a longer time.”
Next up for Xia's tea, is to study catalytic systems with different compositions, such as aluminum oxide rather than silica sheathing.
Platinum is so expensive, Xia says, the converters are sometimes stolen and it is economic to recycle old ones to recover the precious metal. The goal of his research, however, is to use much less platinum, so cars cost less and more of the metal is available for other uses.
Provided by Washington University in St. Louis