February 9, 2009 weblog
Carbon Nanotubes Make Fuel Cells Cheaper

(PhysOrg.com) -- As fuel cells are becoming more popular due to their potential use in applications such as hydrogen-powered vehicles, auxiliary power systems, and electronic devices, the need for the precious metal platinum is also increasing. In fuel cells, platinum is often used as the catalyst for oxygen reduction by splitting oxygen molecules into oxygen ions. However, platinum is rare and expensive: in a fuel cell for a typical car, the platinum catalyst costs about $4,000.
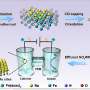
Now, researchers from the University of Dayton have showed that carbon nanotubes can replace platinum as the catalyst in fuel cells, which could significantly reduce fuel cells' overall cost. Carbon nanotubes could even have advantages over platinum, since they could be less resistant to corrosion.
The Dayton researchers, led by Liming Dai, doped an array of nanotubes with nitrogen (VA-NCNTs) to prevent the carbon from reacting with oxygen and forming carbon monoxide (CO). Without the nitrogen, CO would build up on the surface and shorten the catalyst's lifetime. With the nitrogen, the nanotubes are more resistant to this carbon monoxide corrosion and have the potential for long-term operation.
The researchers have not built a complete prototype of a fuel cell with nitrogen-containing carbon nanotubes, and they have not estimated the cost to produce them. However, since carbon is abundant and cheap compared with platinum, the overall cost of the proposed design would likely be much less expensive. Hopefully, the metal-free catalyst will assist researchers in moving fuel cell technology forward.
More information: Kuanping Gong et al. (2009) "Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction." Science Vol. 323. no. 5915, p. 753 doi: 10.1126/science.1166510.
via: Ecogeek
© 2009 PhysOrg.com