New kind of fuel cell delivers energy and fine chemicals with no waste from renewable raw materials
(PhysOrg.com) -- The concept of converting renewable raw materials so cleverly that the same process simultaneously produces both energy and industrially desirable chemicals has been high on the wish-list for those who seek environmentally friendly and resource-saving chemistry. The process should also not release any carbon dioxide.
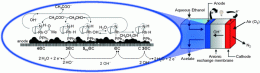
In the journal Angewandte Chemie, Hansjorg Grutzmacher, Francesco Vizza, and Claudio Bianchini and their co-workers from the ETH in Zurich (Switzerland) and the Consiglio Nazionale delle Ricerche (CNR) in Sesto Fiorentino (Italy) have now introduced a new kind of fuel cell: an organometallic fuel cell that efficiently converts alcohols and sugars into carboxylic acids.
Differing from established alcohol fuel cells—the direct alcohol fuel cell and the enzymatic biofuel cell—the organometallic fuel cell (OMFC) works in a completely different way. The secret behind its success is a special molecular complex of rhodium metal that functions as an anode catalyst. The scientists deposited the complex onto a carbon powder support. The interesting thing is that the active catalyst forms during the chemical reaction, and changes step-by-step throughout the catalytic cycle. In this way, a single metal complex forms different catalysts that are each specific for an individual reaction step: the conversion of an alcohol (e.g. ethanol) into the corresponding aldehyde, making the aldehyde into the corresponding carboxylic acid (e.g. acetic acid), and transferring protons (H+) and electrons. As well as alcohols, this system can also convert sugars such as glucose in the same way.
The researchers hope that their new approach could turn out to be a breakthrough in fuel-cell technology. A particular advantage of their new method is that molecular metal complexes are soluble in various solvents, which allows them to be very finely dispersed over very small surfaces. In addition, they provide a very high power density. This could be a way to further miniaturize fuel cells for use as power sources for biological applications like heart pacemakers and biosensors, as well as for the in-vivo monitoring of metabolic processes.
Through the right combination of a tailored molecular catalyst structure and a suitable support material, it could be possible to develop future fuel cells that very selectively convert starting materials with multiple alcohol groups into valuable premium chemicals without the generation of waste materials. This task is very difficult to accomplish by traditional methods.
More information: Hansjorg Grützmacher, et al. A Biologically Inspired Organometallic Fuel Cell (OMFC) That Converts Renewable Alcohols into Energy and Chemicals, Angewandte Chemie International Edition 2010, 49, No. 40, 7229-7233, dx.doi.org/10.1002/anie.201002234
Provided by Wiley