Researcher develops carbon dioxide-free method of producing iron
(PhysOrg.com) -- George Washington University Professor Stuart Licht has developed a revolutionary carbon dioxide-free method of producing iron that could provide a breakthrough for an industry that has been using the same polluting process of iron smelting for more than three thousand years.
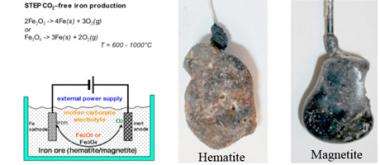
By using renewable solar energy and a process of solar conversion that he patented called Solar Thermal Electrochemical Photo (STEP) energy conversion, Dr. Licht is able to easily extract pure metal iron from the two prevalent iron ores, hematite and magnetite, without emitting carbon dioxide. Today, the commercial iron industry emits an estimated 6.8 trillion tons of carbon dioxide into the atmosphere each year.
“STEP is a new renewable energy process that can capture carbon and makes the materials that society needs without emission of carbon dioxide. We’re developing processes to return the atmosphere to pre-industrial levels of carbon dioxide,” said Dr. Licht.
The process of producing iron free of carbon dioxide emissions is a culmination of more than 20 years of research by Dr. Licht. Through his years of study, Dr. Licht came to understand the efficient use of sunlight and the chemistry of iron, and found that iron ore at high temperatures is significantly more soluble than previously thought. In his most recent research, Dr. Licht found a new way to use electrolysis - a process that uses electricity rather than chemicals to create a reaction - to covert iron ore to iron metal. This high temperature electrolysis requires little energy, and can be powered through conventional or renewable energy sources to reduce or completely eliminate CO2 emissions. When powered by STEP, the electrolysis process is carbon dioxide free, creating no global warming gas emissions when converting the ore into metal. By using both solar thermal energy and visible sunlight, the STEP process converts more solar energy than the best solar cells, as it uses excess solar heat (energy discarded by solar cells) to drive iron production.
Dr. Stuart Licht is a chemistry professor at the Columbian College of Arts and Sciences at the George Washington University. He is an expert in renewable energy chemistry, physical and analytical chemistry. Dr. Licht first presented the STEP process and demonstrated that it can efficiently capture carbon in the article “A New Solar Carbon Capture Process: Solar Thermal Electrochemical Photo (STEP) Carbon Capture” published in the July 14, 2010 issue of "The Journal of Physical Chemistry Letters."
His work with the STEP process is ongoing. Dr. Licht is currently working to develop solar jet fuel and synthetic diesel as well as producing bleach free of carbon dioxide emissions. The iron study was performed at the Licht laboratories at GW together with Dr. Baohui Wang, a visiting professor from the Northeast Petroleum University in China, and was published in the August 23, 2010, online edition of "Chemical Communications."
Provided by George Washington University