Popping Bubbles Hold Promise in Cellular Drug Injection
(PhysOrg.com) -- A new technique that harnesses the power of mighty microscopic bubbles, developed by Duke engineers, can open for a blink of the eye nanometer-sized entries into individual cells.
The same process that crumbles kidney stones and rusts ship propeller blades may hold the key to successfully injecting drugs directly into individual cells without harming them.
A new technique that harnesses the power of mighty microscopic bubbles, developed by Duke University engineers, can open for a blink of the eye nanometer-sized entries into individual cells.
Scientists have long known that miniscule bubbles in a liquid possess a great deal of useful energy, and when they collapse -- a process known as inertial cavitation -- for the briefest of moments, the potential energy stored in the bubble can be focused and released in a number of ways. That includes shock waves or heat (with temperatures equal to that of the surface of a star produced inside a collapsing bubble) or tiny high-speed spurts of liquid that can destroy cells by bursting them open.
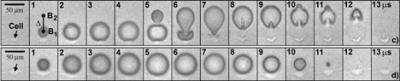
The Duke engineers have demonstrated in a new set of experiments that collapsing two of these microscopic bubbles in tandem, one right after the other, creates a "microjet" capable of tearing a tiny pore on the surface of the cell. This pore formation is transient to allow drugs to enter the cell while the cell membrane will seal itself fast enough to keep the contents of the cell spilling out.
Pei Zhong, associate professor of mechanical engineering and materials science at Duke's Pratt School of Engineering, said this could become a valuable technique to not only safely introduce drugs into individual cells, but also genetic materials, such as genes and siRNAs, into cells to help treat patients with cancers, heart disease and hereditary disorders.
"The controlled creation of a second bubble in close proximity as the first bubble collapses produces a unique bubble-bubble interaction that forms a potent microjet of liquid, which creates a pore on the cell membrane without destroying the cell," said Zhong. "This microjet is significantly forceful with more focused energy than its counterpart created by a single bubble."
Zhong and Pratt research scientist Georgii Sankin published the results of their experiments in the journal Physical Review Letters this month.
In addition to developing this novel one-two bubble punch approach, the Duke researchers also for the first time captured the entire process of bubble-cell interaction and subsequent drug uptake with high-speed cameras.
For their experiments, the scientists grew breast cancer cells in a microfluidic channel, in which the liquid solution was colored by blue dye and then two precisely aligned laser beams were fired into the liquid next to the cell. The microjet is generated by the mutual interaction between the collapsing first bubble and the expanding second bubble.
Zhong said the process is analogous to a child swinging. When someone pushes an already swinging child, it gives that child an extra boost to swing higher and faster. That is in essence what the expansion of the second bubble causes -- transfer of energy and momentum to the first bubble.
"When we looked at the cells immediately after the laser exposure, we saw that the blue dye had entered the cell through a pinpoint rupture at the microjet impact site and gradually diffused into the inside of the cell, but the rest of the cell membrane remained intact," Zhong said.
The size of the pore varies from a few hundred nanometers to a few microns, sufficient to allow drugs, DNA or other macromolecules to be infused into cells without damaging them, Zhong said. He envisions a time when cells, such as immune or stem cells, could be removed from a patient, new genetic materials inserted into the cells, and the cells being reinjected back into the patient, such as in ex vivo therapies.
In an editorial accompanying this issue of the journal, Claus-Dieter Ohl, from the Nanyang Technical University and the Institute of High Performance Computing, both in Singapore, wrote that the Duke experiments were "... to my knowledge the most complete experiment that characterizes the flow, measures the uptake on single cells, and obtains a one-to-one correlation between the observed jet and the measured membrane pore.
"The Duke team's method qualifies as a new technique for drug delivery to single cells, and if engineered further may become a tool for biologists for particularly delicate cell lines and perhaps stem cells," Ohl concluded.
Zhong, who also researches ways to improve minimally invasive or non-invasive treatments of various diseases, such as cancers and urinary tract stones, said their new methodology and experimental technique can provide deeper insights into the mechanisms of cavitation-generated bioeffects at the cellular level, which is key for ensuring the success of therapeutic ultrasound applications in clinic medicine.
"Understanding this process should help us design better medical devices," he said.
Provided by Duke University

















