Hijacked supplies for pathogens: Legionnaire's disease bacteria tap into the material transport in immune cells
(PhysOrg.com) -- When it infects the lungs, the Legionnaire’s bacterium Legionella pneumophila causes acute pneumonia. The pathogen’s modus operandi is particularly ingenious: it infiltrates deliberately into cells of the human immune system and injects a host of proteins which then interfere in the normal cellular processes.
Scientists from the Max Planck Institute of Molecular Physiology in Dortmund have now discovered how Legionella reprogrammes the cells to ensure its own survival and to propagate. They examined a protein used by the pathogen to divert the material transport within the cells for its own purposes. (Science, July 22, 2010)
During a Legionella infection, the bacteria are engulfed by immune cells and bound by a membrane in the cell interior. Legionella protects itself against destruction by releasing proteins that reprogramme the human cell and exploit it for its own purposes. One of these proteins is DrrA. Previous studies succeeded in demonstrating that DrrA diverts the material transport in human cells in the direction of the pathogen, using what are known as Rab proteins for this purpose.
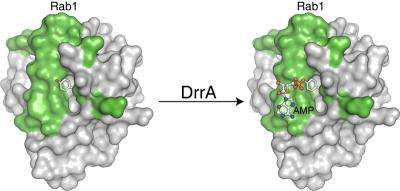
Rab proteins are switch molecules that coordinate transport vesicles within cells. In this capacity, they ensure that these membrane-bound vesicles reach the correct destination at the right time. Of the total of 60 different Rab proteins, DrrA specifically uses the Rab1 molecule for its own purposes: it deposits Rab1 on the membrane enclosing the bacteria and activates it. As a result, part of the material transport of the human cell is diverted to the vesicle containing the bacterium.
The structural and biochemical analysis of DrrA led the Dortmund-based scientists to make an astonishing discovery: DrrA is not only capable of activating Rab1, it also appears to be able to extend its activated state. To this end, DrrA blocks the switching-off of Rab1 and the necessary recognition site for regulatory proteins by attaching an AMP molecule to Rab1. "The permanent activation of Rab1 by DrrA could ensure increased material transport in the direction of the Legionella containing compartment and hence support its survival," concludes Aymelt Itzen from the Max Planck Institute of Molecular Physiology.
"These results represent an example of how the molecular analysis of bacterial diseases can help us not only to understand the cellular mechanisms involved in an infection, but also the functioning of healthy cells," explains Roger Goody from the Dortmund Institute. In the case of Legionnaire’s disease, the study of the bacterial protein DrrA reveals how a human regulatory protein (Rab1) is activated in a targeted way and maintained in an active state. This raises the question as to whether Legionella devised this kind of regulation or whether healthy cells can also control material transport in a similar but hitherto unknown way.
More information: M.P. Mueller, H. Peters, J. Bluemer, W. Blankenfeldt, R.S. Goody, A. Itzen, The Legionella Effector Protein DrrA AMPylates the Membrane Traffic Regulator Rab1b, Science, July 22, 2010 / DOI: 10.1126/science.1192276
Provided by Max-Planck-Gesellschaft