Biologists reveal structure of cell nucleus 'gatekeeper'
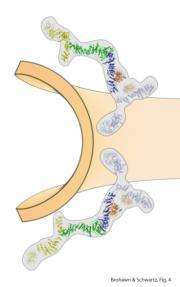
(PhysOrg.com) -- Biologists led by associate professor Thomas Schwartz (MIT) have worked out a rudimentary architectural plan for the nuclear pore complex (NPC), the gatekeeper of the cell's nucleus.
The massive NPC, which allows molecules such as RNAs and proteins, to move in and out of the nucleus, is one of the largest macromolecular structures in the cell (containing about 5 million atoms). Its structure has been considered a "holy grail" in structural biology for many decades. Schwartz and his team found that the NPC scaffold forms an open lattice structure, similar to another membrane coating complex, called COPII. That complex has far fewer components and is involved in a completely different cellular process, called vesicular transport, by which materials move in, out and around the cell enclosed in vesicles.
The finding reveals a remarkable evolutionary story. The proteins that form the building blocks in the NPC and the COPII vesicle coats are so unique that they still have not been identified anywhere else in the cell. Once the models of the proteins were complete, it became clear that these two cellular structures share a common ancestor, dating back over one billion years, which already contained the same building blocks connected in very similar fashion. Second, discovering the specific relationship between the NPC and the much simpler, better understood COPII vesicle coat constitutes a significant step toward understanding the entire NPC assembly, which appears to be remarkably modular and most likely self-assembling.
Graduate student Stephen Brohawn created a complex of three nuclear pore proteins in bacteria and grew microscopic crystals of the complex. Then he solved the structure of the complex by X-ray crystallography. In general, complexes as large as this, especially nuclear pore proteins, are notoriously difficult to crystallize and solve the structure of, but strategies such as solving the structure of small pieces of the complex separately made the problem tractable.
Understanding structurally that the NPC and the COPII vesicle coat are evolutionarily related opens up a wide avenue of future research. The structures available now are still only pieces within the entire nuclear pore complex puzzle. However, unraveling the common architecture now serves as a guide to employ the next steps toward understanding the entire transport machine. Also, several other ideas about the NPC architecture that were largely speculative can now be put to rest.
"A complete structure of the NPC is the project of a lifetime, but we have shown that it is feasible and worth it, and that many unexpected discoveries can be made on the road as well," says Schwartz.
More information: "Molecular architecture of the Nup84-Nup145C-Sec13 edge element in the nuclear pore complex lattice," Stephen Brohawn and Thomas Schwartz. Nature Structural & Molecular Biology, Oct. 25, 2009.
Provided by Massachusetts Institute of Technology (news : web)

















