Stretching the Golgi: a link between form and function
A research team at the University of California, San Diego School of Medicine has provided a surprisingly simple explanation for the mechanism and features of the "Golgi apparatus" - a structure that has baffled generations of scientists. The model developed by the UC San Diego scientists suggests that the Golgi's unusual shape is a direct consequence of the way it works. Their study will be published in the October 16 issue of the journal Cell.
The Golgi apparatus serves as a processing center for the exportation of proteins, lipids and other large molecules to their final destinations outside of the cell.
"Its primary function is to serve as a way station for extracellular protein traffic," said principal investigator Seth J. Field, assistant professor of medicine at UC San Diego. "Much of our body is made up of material exported by cells - for example, antibodies, hormones, growth factors, even much of the material that makes up bone, cartilage, skin, and hair - and they all depend on the Golgi apparatus working correctly."
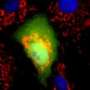
The Golgi apparatus is made up of flattened, membrane-bound stacks called cisternae, but the reason for their shape and structure have been unclear since the Golgi was first identified by Nobel Prize winner George Palade and colleagues using an electron microscope more than 50 years ago. Each cisterna is made up of a flattened disk that carries enzymes meant to help or modify the protein cargo that travels through them.
Golgi membranes, from yeast to human cells, rely on a particular type of lipid signaling molecule - phosphatidylinositol-4-phosphate, or PtdIns(4)P - for normal trafficking. Field was a 2008 winner of the NIH New Innovator award to study the function of a group of these lipid signaling molecules called phosphoinositides, which are known to play critical roles in regulating cell growth and death, metabolism, and communication processes within cells. His search to understand the function of these molecules led to what he described as an unexpected discovery about how PtdIns(4)P contributes to the structure of the Golgi.
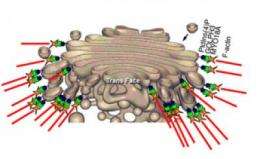
Using a proteomic lipid binding screen, Field and colleagues identified a particular Golgi protein, GOLPH3 which links to PtdIns(4)P and to a contractile protein similar to those found in muscle called MYO18A. They discovered that this three-way interaction applies a tensile force that is required for efficient formation of the tubules and vesicles necessary for extracellular transportation.
Their research suggests that another consequence of this tensile force is to stretch the Golgi into the extended ribbon observed by fluorescence microscopy and the familiar flattened form observed by electron microscopy.
"The NIH New Innovator Program is an effort to find exceptionally creative approaches to major challenges in biomedical and behavioral research," said NIH Director Francis S. Collins, MD, PhD. "Dr. Field has fulfilled that promise with an extraordinary insight into the origin of a structure and a process that has perplexed biologists for decades. He has shown that an interaction of three particular proteins is essential for Golgi apparatus function and, remarkably, that this interaction also generates the force that shapes the apparatus into the flattened ribbons first observed by electron microscopy half a century ago. He has given us considerably deeper insight into the mechanism of the Golgi apparatus and a stunning example of the linkage between form and function."
Source: University of California - San Diego (news : web)