Study shows how disruption of spectrin-actin network causes lens cells in the eye to lose shape

A network of proteins underlying the plasma membrane keeps epithelial cells in shape and maintains their orderly hexagonal packing in the mouse lens, say Nowak et al. The study will appear in the September 21, 2009 issue of the Journal of Cell Biology (online September 14).
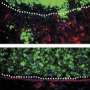
Spectrin, F-actin, and associated proteins form a meshwork that supports and shapes the plasma membrane of red blood cells. A similar network underlies the membranes of other cell types, including lens fiber cells: elongated epithelial cells that encircle vertebrate lenses in concentric layers, appearing in cross section as tightly packed hexagons. Actin filaments within this membrane skeleton are stabilized by their association with members of the tropomyosin and tropomodulin families of actin-binding proteins.
In mice lacking tropomodulin1, gamma-tropomyosin was also lost from the membrane skeleton of lens fiber cells. F-actin and spectrin remained associated with the cell membrane, but gaps appeared in the usually continuous protein network, suggesting that the two actin-binding proteins stabilize a subset of actin filaments required to link the network together.
Scanning electron microscopy revealed that fiber cell membrane protrusions, which interlock with neighboring cells, were distorted and irregularly arranged in the absence of tropomodulin1. And although the fiber cells appeared hexagonal when first forming at the lens' equator, they often became misshapen and disorganized as they matured and moved toward the lens' center.
Senior author Velia Fowler thinks that disruption of the spectrin-actin network alters the adhesive interactions between neighboring cells, causing their shapes and packing to become disordered in response to the mechanical stresses associated with lens growth and eye movements.
More information: Nowak, R.B., et al. 2009. J. Cell Biol. doi:10.1083/jcb.200905065.
Source: Rockefeller University (news : web)