Controlling the size of nanoclusters
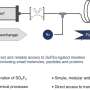
Melissa Patterson, a W. Burghardt Turner Fellow at Stony Brook University (SBU), will give a talk at the American Chemical Society's national meeting in Philadelphia on controlling the size of nanoclusters, research she performed using a new instrument at the U.S. Department of Energy's Brookhaven National Laboratory. Built by Brookhaven Lab and SBU scientists, the instrument enables researchers to make nanoclusters of 10 to 100 atoms with atomic precision.
Patterson and her Brookhaven colleagues created model nanocatalysts of molybdenum sulfide, the first step in developing the next generation of materials to be used in hydrodesulfurization, a process that removes sulfur, a pollutant, from natural gas and petroleum products. They made size-selected molybdenum sulfide nanoclusters as gaseous ions, and deposited them on a gold surface, which interacts weakly with the gold support, leaving the nanoclusters intact.
"We learned that even though we were using the same molecule — all were composed of molybdenum and sulfur — size and structure is important in determining reactivity," Patterson said. "The most reactive nanocluster of those that we tested had six atoms of molybdenum and eight atoms of sulfur. It readily absorbed sulfur and let go of carbon monoxide, which makes it an effective catalyst."
Brookhaven Lab chemist Michael White and Brookhaven research associates YongMan Choi and Ping Liu collaborated with Patterson on this work. DOE’s Office of Basic Energy Sciences, within the Office of Science, funded this research through the Nanoscale Science, Engineering and Technology Initiative.
Patterson’s talk, titled “Size-selected deposition of transition metal sulfides: Insights toward model systems in catalysis,” is scheduled to be given on Tuesday, August 19, at the Pennsylvania Convention Center.
Provided by Brookhaven National Laboratory