Scattering of hydrogen makes calculation easier
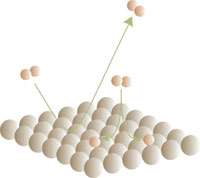
The chemical reaction of hydrogen molecules (H2) with a platinum surface can be calculated much more straightforwardly than many researchers to date had thought. This is encouraging for research into hydrogen as a clean fuel and heterogeneous catalysis, which is where the reactions of molecules to metal surfaces plays a significant role. Chemists can now test theories on a broad scale which describe the interaction of molecules with metal surfaces.
The Leiden theoretical chemists Ernst Pijper, Roar Olsen and Geert-Jan Kroes, together with colleagues from Amsterdam and from Spain, demonstrated that the Born-Oppenheimer approach can be used in predicting the reaction of hydrogen molecules with a metal surface. This is an approach which considerably simplifies chemical calculations by splitting them in two. A heated debate is currently raging on the applicability of the Born-Oppenheimer approach to reactions of molecules with a metal surface - a class of reactions which is very important in this application.
The researchers published their findings last Thursday on the site of Science Express. This site publishes on the web a number of what they call hot results, sometimes weeks before they appear in the journal Science. The Born-Oppenheimer approach can be used, according to the researchers in their article, because a hydrogen molecule which is fired at a platinum surface, first splits into two atoms or is scattered at the surface before any strong interaction with the platinum takes place.
Chemical reactions are generally so complicated that it is difficult to make predictions using quantum mechanical calculations, the specialism of theoretical chemistry. The Born-Oppenheimer approach is a useful tool for making these chemical calculations.
Born and Oppenheimer in 1927 postulated the idea that you can split a chemical calculation in two. First you solve the movement of the electrons and then the movement of the atomic nuclei which are so much heavier and slower than electrons that you can imagine they are standing still in comparison.
This makes the calculation much easier, and the Born-Oppenheimer approach is then also a welcome tool for predicting the progress of chemical reactions of complex systems. It works particularly well in calculating the behaviour of many gas phase reactions.
The Born-Oppenheimer approach can only be applied if the reaction processes are adiabatic, which means that the electrons follow the movements of the nuclei and do not get out of step. And a lot of reactions of molecules with a metal surface are not adiabatic.
It has been shown for a number of molecules investigated to date which have been fired at a metal surface, that they already form a strong bond with the surface before they dissociate or are scattered. The consequence is that the molecules affect the metal surface, by forming so-called electron-hole pairs. This process is non-adiabatic; the electrons no longer follow the movement of the nuclei, and the Born-Oppernheimer approach cannot therefore be used.
Other molecules, like nitrogen, which when stretched like to take up electrons, can, if they are fired in a highly excited state at a metal surface that weakly binds electrons, shoot electrons out of a metal surface. This case also constitutes a breakdown of the Born-Oppenheimer approach.
But, based on calculations and experiments, the three Leiden researchers and their colleagues have been able to show that hydrogen molecules behave very differently if they are fired at a metal surface.
At first, hydrogen molecules split into two atoms, or are back-scattered to the platinum surface before they form the strong bond with the metal whereby electron-hole pairs can be formed.
Secondly, a hydrogen molecule has a low electron affinity, which means that it does not readily take up an electron. Therefore, it does not tend to 'take' an electron of the metal far from the surface. So, here too there is no risk of forming electron-hole pairs.
The reaction of molecular hydrogen with a platinum surface, and the scattering of the molecule on the surface is then an adiabatic process, and the Born-Oppenheimer approach can readily be applied to this class of reactions.
‘It is not the case that hydrogen economy will be a fact next year as a result of this discovery,’ says Prof. dr. Geert-Jan Kroes, who is conducting fundamental research into hydrogen as a source of clean energy, and who transformed his faculty into a hydrogen plaza on the Science Day in 2005. 'But it is encouraging, because some hydrogen storage systems are based on the disassociation of hydrogen, that is the breaking of the hydrogen-hydrogen bond. Sometimes a metal is added, such as palladium. And disassociation can be described with the Born-Oppenheimer approach. Platinum is not a candidate for promoting the storage of hydrogen. We do know now that a whole class of reactions, particularly dissociation of hydrogen on metal surfaces, can be solved simply, so that we can conduct large scale testing of theories which describe the interaction of molecules with metal surfaces.‘
Source: Universiteit Leiden