This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A new method for exploring the hyperpolarization of hydrogen
Nuclear magnetic resonance (NMR) is a powerful tool that is widely used in many scientific fields, from analytical chemistry to medical diagnostics. However, regardless of its widespread use, there are still areas in which this very informative method cannot be employed because it is limited by its low sensitivity.
Therefore, many efforts are being made to increase its sensitivity. One of the methods that is capable of enhancing NMR signals is a method called parahydrogen-induced polarization, which uses the unique property of one of the isomers of hydrogen molecules called parahydrogen, which can induce strong NMR signals in other molecules, including biologically relevant ones.
Recently, researchers at the Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) delved into the mystery of the fate of parahydrogen molecules associated with hyperpolarization, and they observed that parahydrogen molecules can be converted into orthohydrogen, which has an unusual NMR signal. The research presented here is a step forward in the study of hydrogen isomers.
Nuclear magnetic resonance (NMR) makes it possible to analyze the structures of even very complex molecules. Its fundamentals are based on the investigation of the behavior of the magnetic properties of nuclei, whose properties manifest themselves as magnetic moments of nuclei in atoms in the presence of a strong magnetic field.
This interaction is, however, weak, and thus the investigation of this interaction is very difficult and requires expensive scientific equipment. In short, NMR is a very insensitive method.
Therefore, researchers have been trying to enhance NMR sensitivity, and one of the most compelling methods to accomplish this utilizes the unique properties of hydrogen molecules. This molecule can exist in two forms: orthohydrogen (o-H2), with two spins oriented in the same direction, and parahydrogen (p-H2), with two spins oriented in the opposite direction.
The uniqueness of parahydrogen molecules lies in the fact that their spin orientation, under specific conditions, can be utilized for NMR signal enhancement in other molecules. These specific conditions can be achieved via protocols in which parahydrogen interacts with other molecules, and this interaction is mediated by a catalyst.
Because of this interaction, the NMR signal in the interacting molecules is enhanced. However, during this interaction, p-H2 spins are reoriented, and o-H2 is created. In some cases, this conversion can lead to the creation of a very specific orthohydrogen molecule, which, when it is detected by NMR, its signal is manifested as a Partially Negative Line (PNL).
Despite several reports in the literature mentioning the recording of PNL, its nature remains unexplained and is widely treated as an artifact that requires more in-depth investigation.
Recently, researchers from the Institute of Physical Chemistry at the Polish Academy of Sciences, led by Prof. Tomasz Ratajczyk, in collaboration with researchers from the Institute of Physical Chemistry at the Technical University of Darmstadt and the Faculty of Chemistry at the University of Warsaw, have focused on this issue, and have invented a simple procedure that can be used for the generation of PNL signals.
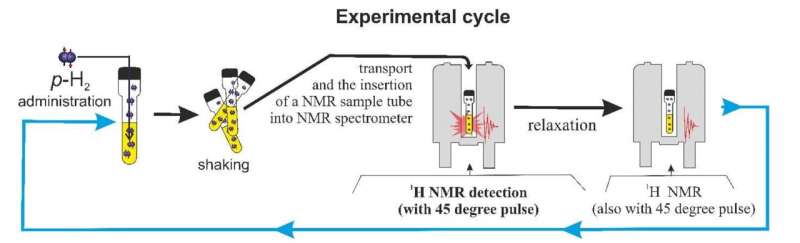
They discovered that PNL could be initiated in SABRE when simple ligands such as pyridine (Py) and dimethylsulfoxide (DMSO) are used, and this can be done with the simple iridium-based N-Heterocyclic Carbene (NHC) complexes used as catalysts. The experiments were performed in three deuterium-labeled solvents: methanol-d4, acetone-d6, and benzene-d6.
In their work described in Angewandte Chemie International Edition, they focused on the determination of the conditions that are necessary for the generation of PNL, presenting a hypothesis concerning the occurrence of such an effect as a prelude to further mechanistic studies of PNL.
"We decided to closely examine the interplay between the activation processes and the occurrence of PNL to hypothesize which transient species can be potentially concerned with uncommon PNL signals," says Prof. Tomasz Ratajczyk
They recorded the PNL signal during the activation process of the catalyst, where the hyperpolarization of the ligands was increasing, and the intensity of the PNL signal was increasing, reaching a maximum, and then decreasing. Researchers discovered that the appearance of PNL is connected with the chemical processes that occur during pre-catalyst activation. By using a few solvents, they also discovered that PNL can be observed better when the activation process is slower.
The presented studies determined the specific conditions needed to easily induce the PNL effect using common hyperpolarization with the SABRE protocol for simple molecules like Py or DMSO, as well as conditions without any ligands.
They also found an interesting relationship between PNL intensity and the SABRE hyperpolarization of Py and DMSO. It was noticed that the effect is present only during the initial hyperpolarization stage and fades with the progress of hyperpolarization efficiency.
The unusual and uncommon signal during NMR studies can be a key point of research that can be used to investigate heretofore unknown hyperpolarization mechanisms.
Prof. Tomasz Ratajczyk adds, "We have also noticed an interesting correlation between the strength of the PNL effect and the efficiency of the SABRE hyperpolarization of Py and DMSO. More precisely, the PNL effect is present only during the activation stage, i.e., when hyperpolarization is not fully operating in the sample."
"The understanding of the conditions in which the PNL effect can be observed in a reproducible manner will facilitate a more thorough comprehension of the basic aspects of the SABRE mechanisms, which are crucial for the efficient hyperpolarization of biorelevant systems."
Hydrogen is one of the most widely studied molecules, which has resulted in its chemistry being well understood. It can be used for studies of many compounds, making it a powerful tool in the investigation of many mechanisms and finding applications even in biomedicine.
Nevertheless, some aspects of hydrogen chemistry still remain a mystery, and its properties can be quite surprising. The findings related to its utilization in hyperpolarization in NMR, which were discovered by researchers from IPC PAS, still need to be investigated further in order to determine the mechanisms behind the PNL signal. The results clearly show the importance of remaining curious, even about some things that are apparently well-understood.
More information: Marek Czarnota et al, A Straightforward Method for the Generation of Hyperpolarized Orthohydrogen with a Partially Negative Line, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202309188
Journal information: Angewandte Chemie International Edition
Provided by Polish Academy of Sciences