This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Green hydrogen: Improving the stability of iridium catalysts with titanium oxides
Anodes for the electrolytic splitting of water are usually iridium-based materials. In order to increase the stability of the iridium catalyst, a team at HZB and a group at HI-ERN have now produced a so-called material library: a sample in which the concentration of iridium and titanium oxides is systematically varied.
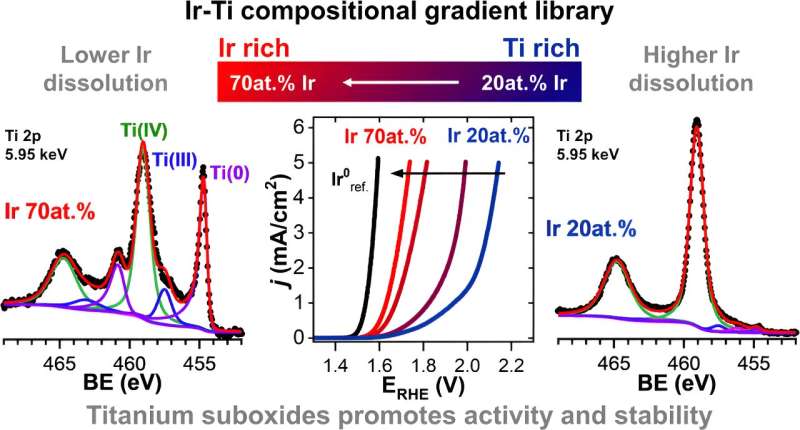
Analyses of the individual sample segments at BESSY II in the EMIL laboratory showed that the presence of titanium oxides can increase the stability of the iridium catalyst significantly.
One option for storing energy from the sun or wind is the production of "green" hydrogen by electrolysis. Hydrogen stores energy in chemical form and releases it again when burnt, producing no exhaust gases, only water. Today, iridium is the state-of-the-art catalyst for this reaction. However, iridium increasingly dissolves in the acidic environment of the electrolysis cell so that the catalytic effect quickly wanes.
"We wanted to investigate whether the stability of the catalyst can be improved by adding different proportions of titanium oxide," says Prof Dr. Marcus Bär (HZB). Although titanium oxide is not catalytically active, it is very stable. "We had some indications that the presence of titanium oxide would have a positive effect on stability without influencing the catalytic effect of the iridium. But we also wanted to find out whether there is an ideal mixing ratio."
The sample as a materials library
The sample was produced at the Helmholtz Institute Erlangen-Nuremberg for Renewable Energies (HI-ERN) by Prof Dr. Olga Kasian's team by sputtering titanium and iridium with locally varying compositions. It is a so-called thin-film materials library on which the iridium content varies from 20% to 70%
At BESSY II; the team used X-ray spectroscopic methods to analyze how the chemical structure changes depending on the iridium content of the mixed iridium-titanium oxide samples. Several effects played a role here: for instance, the presence of titanium suboxides (such as TiO and TiOx) improved the conductivity of the material.
Another exciting result was that some of the titanium oxides dissolve faster in the aqueous electrolyte than iridium, creating micropores on the surface. This promoted the oxygen evolution reaction because more iridium atoms from the lower layers came into contact with the electrolyte.
The main effect, however, is that titanium oxides (TiO2, as well as TiO and TiOx) significantly reduce the dissolution of iridium. "In the sample with 30 % titanium added compared to a pure iridium electrode material, we saw an iridium resolution that was approximately 70 % lower," says Marianne van der Merwe, who carried out the measurements as part of her doctorate with Marcus Bär.
High relevance for practical use
But how relevant are such results from laboratory research for industry? "If there are already established technologies, it's always difficult to change anything at first," says Marcus Bär. "But here we show how the stability of the anodes can be significantly increased with a manageable amount of effort."
The study is published in the journal ACS Catalysis.
More information: Marianne van der Merwe et al, The Chemical and Electronic Properties of Stability-Enhanced, Mixed Ir-TiOx Oxygen Evolution Reaction Catalysts, ACS Catalysis (2023). DOI: 10.1021/acscatal.3c02948
Journal information: ACS Catalysis
Provided by Helmholtz Association of German Research Centres