This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers develop new electrochemical chemosensor for fast, effective diagnosis of a lethal pulmonary disease
Patients struggling with some chronic diseases often must wait years for a proper diagnosis. For example, symptoms such as shortness of breath can be attributed to many pulmonary as well as cardiovascular disorders, so patients may be treated for a misdiagnosed disease that is far from accurate diagnosis and treatment.
Therefore, one of the most promising methods to deal with this problem is to track the levels of specific compounds in the body during the development of a specific disease. Moving in this direction, scientists at the Institute of Physical Chemistry of the Polish Academy of Sciences (Warsaw, Poland) and the National Kaohsiung University in Kaohsiung (Kaohsiung, Taiwan) presented their research on developing a method for fast and effective diagnosis of a lethal pulmonary disease.
Idiopathic pulmonary fibrosis (IPF) is a chronic disease causing lung fibrosis with fatal results, leading to suffocation. Its most common symptoms are dry cough and shortness of breath, which can be associated with multiple disorders. So, it can often be misdiagnosed as many different diseases, making accurate diagnosis long and arduous, dramatically affecting a patient's quality of life.
Moreover, symptoms can be delayed until it is too late to treat the patient successfully. IPF development is still a medical mystery. Hence, there is a tremendous need for early diagnosis of IPF. Electrochemical detection of IPF biomarkers is one of the solutions. Biomarkers are specific compounds, e.g., proteins, nucleic acids, or other compounds abnormally produced by the body during disease development. For IPF, several biomarkers can be distinguished.
One of them is matrix metalloproteinase-1 (MMP-1), which degrades fibrillar collagens in the respiratory tract. Despite the well-known chemical properties of MMP-1, rapid monitoring of this biomarker in bodily fluids as part of IPF progression is now a far cry from a dream diagnosis.
Recently, researchers from the Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) in Warsaw (Poland), in collaboration with scientists from the Department of Chemical and Materials Engineering at National Kaohsiung University in Kaohsiung (Taiwan), targeted fast-track IPF and rapid diagnostics by devising a new electrochemical chemosensor for selective, rapid, and efficient determination of the IPF biomarker, in particular MMP-1.
To prepare this chemosensor, the researchers relied on molecular imprinting in polymers, a technique based on mixing a functional monomer, a cross-linking agent, and a template, followed by generating a polymer matrix that forms (template molecule)-shaped molecular cavities matching a molecular key that fits into the polymer lock.
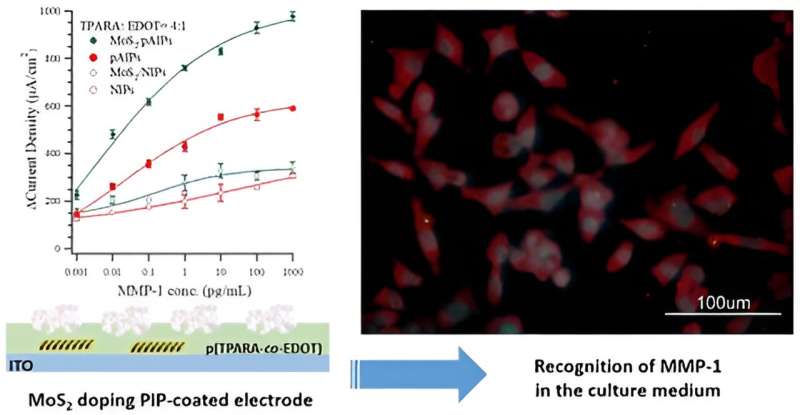
Specifically, they modified the transparent electrode (a glass slide coated with the conducting indium-tin oxide called ITO) with a molecularly imprinted polymer (MIP), poly(TPARA-co-EDOT), made from two monomers, EDOT and TPARA. Additionally, doped with a MoS2-2D flaky material, the MIP was templated with the peptide epitope of the MMP-1 protein biomarker.
Then, this template was removed from the MIP, leaving molecular cavities of a shape and size characteristic of the peptide epitope molecules used for imprinting. Since the cavities match these characteristic peptide molecules, the MIP can easily be used to determine the matching molecule. Intriguingly, doping the MIP with MoS2 enhanced the MMP-1 detection limit significantly compared to the undoped MIP.
Dr. Piyush S. Sharma claims: "Incorporating novel materials into electrochemical chemosensors can enhance their performance and help elucidate their sensing mechanism. In our research, (peptide epitope)-templated MIP was doped with MoS2 flakes with a mean size of 0.6−1.5 μm during its deposition as a thin film onto an ITO electrode. Essentially, this doping doubled the electrochemical response (above the background) to the target MMP-1 protein biomarker."
The MMP-1 macromolecule has several peptides located at its edges, the so-called epitopes, recognized by the immune system. These epitopes can be employed successfully as an imprint in electrochemical MIP chemosensors. Since protein imprinting would not result in their successful determination, leading to large cavities that fit many smaller-molecule compounds, the imprinted molecules were of peptide epitope, much smaller than proteins.
Moreover, in addition to their smaller size, peptides are more stable than proteins under experimental conditions, including using an organic solvent when forming a polymer film on the electrode surface. It is worth mentioning that using MoS2 flakes enables the detection of the MMP-1 biomarker and, hence, idiopathic pulmonary fibrosis.
"The MoS2-doped pAIPs film-coated electrode performance is comparable to the recent literature. Finally, the electrode was used to determine MMP-1 in the culture media of gene-edited HEK293T cells and, compared to a commercial ELISA assay, its accuracy was high," remarks Prof. Włodzimierz Kutner.
This research, detailed in ACS Applied Nano Materials, holds promise for monitoring the development of chronic and progressive diseases of unknown etiology and pathogenesis, including idiopathic pulmonary fibrosis.
There is still room for testing detection under disruptive conditions, but hopefully, molecular imprinting in polymers will gain more attention and application in biomedicine, bringing society closer to rapid and accurate diagnosis of many challenging illnesses. The researchers hope their proven concept of a molecularly imprinted electrochemical chemosensor can also be adapted to other diseases and personalized medicine.
More information: Mei-Hwa Lee et al, MoS2 Nanosheet-Doped Peptide-Imprinted Polymer-Coated Electrodes for Electrochemical Determination of CRISPR/dCas9-Activated Protein Expression, ACS Applied Nano Materials (2023). DOI: 10.1021/acsanm.3c04130
Provided by Polish Academy of Sciences