This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New insights into antihistamine binding could lead to more effective treatments
Even if two molecules have the exact same chemical formula and the same number and types of bonds, their three-dimensional arrangements can still be different. While some people might mistakenly disregard this as a minor detail, even simple changes in the position or orientation of a functional group can dramatically affect the biological properties of a molecule, sometimes rendering an otherwise benign substance into a highly toxic one. Thus, the study of such possible molecular variants, called "geometric isomers," is essential in the field of drug development.
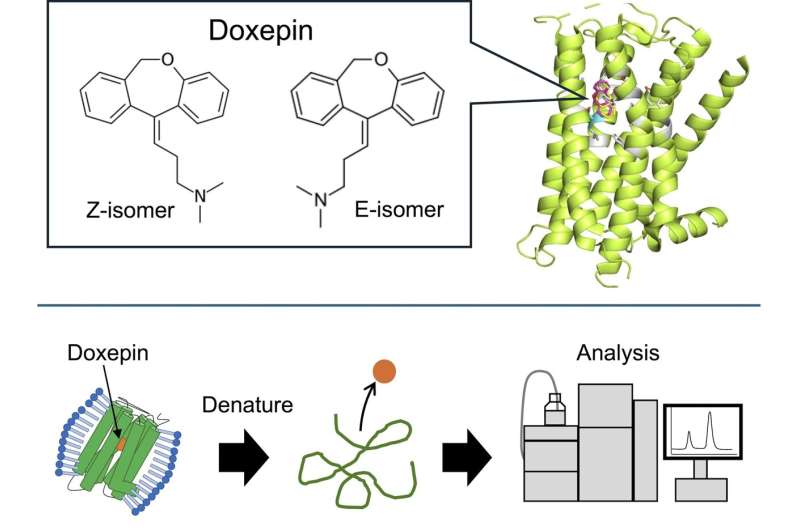
Doxepin stands out as a notable example of a drug that is commercialized as a mixture of two geometric isomers, namely the E- and Z-isomers. Both doxepin isomers bind to histamine H1 receptor (H1R), which is expressed throughout the central nervous system, smooth muscle cells, and vascular endothelial cells.
Besides its use as an antihistaminic drug, doxepin is also typically used as an antidepressant and sleeping aid. While biological tests in animals have shown that the Z-isomer is more effective than the E-isomer, the differences in affinity to H1R between the E- and Z-isomers are unknown. Moreover, the specifics of how these compounds actually bind to H1R remain elusive.
Against this backdrop, a research team from Tokyo University of Science, Japan, set out to clarify the finer details of the interactions between doxepin isomers and H1R. Their latest paper, which was published on June 25, 2024, in the Journal of Molecular Recognition, was co-authored by Professor Mitsunori Shiroishi, Mr. Hiroto Kaneko, and Associate Professor Tadashi Ando, among others. This study is a follow-up to past work done by Prof. Shiroishi and colleagues.
"We previously revealed the crystal structure of the complex formed by H1R and doxepin, but we were unable to determine which isomer was bound," Shiroishi explains. "We then came up with a method to determine the binding affinity of the isomers, and thus carried out this study."
To achieve this challenging goal, the researchers first produced a customized yeast expression vector by strategically inserting the H1R gene into it. This vector was used to modify yeast cultures so that they produce H1R.
After retrieving the membranes from these cells, they applied a solution containing commercial doxepin, producing H1R-doxepin complexes. Following extraction and purification of these complexes, they removed any excess (unbound) doxepin. Finally, by denaturing the H1R receptors, they could free the bound doxepin molecules and measure their numbers in a high-performance liquid chromatography setup.
Using this protocol, the researchers could accurately quantify the amount of each isomer that was bound to the extracted receptors, which is directly tied to their relative binding affinity. They found that the affinity to H1R of the Z-isomer was over five times higher than that of the E-isomer.
The team then delved deeper into the nature of how doxepin isomers bind to H1R. Through experiments on a mutant variant of H1R coupled with molecular dynamics simulations, they revealed that the Thr112 side chain in the ligand-binding pocket of H1R creates a chemical environment that enhances selectivity for the Z-isomer.
Taken together, the findings of this study shed light on how a widely used small molecule drug interacts with an important cellular receptor.
"Our efforts could serve as the basis for designing next-generation antihistamines that are more effective and have fewer side effects," says Shiroishi. "Worth noting, this newfound knowledge will be useful for designing compounds that bind not only to H1R, but also other disease-relevant target proteins."
The rational design of future drugs, aided and validated by computational techniques like molecular dynamics simulations, could usher in a new era in medicine. More specifically, by understanding the binding properties of isomers in detail, many small-molecule drugs could be made more effective, safer, and better suited for targeted therapies.
More information: Hiroto Kaneko et al, Binding characteristics of the doxepin E/Z‐isomers to the histamine H1 receptor revealed by receptor‐bound ligand analysis and molecular dynamics study, Journal of Molecular Recognition (2024). DOI: 10.1002/jmr.3098
Provided by Tokyo University of Science