April 7, 2016 report
Removing intercalated water from nitrogen-doped graphene-oxide sheets
(Phys.org)—Fuel cells require a catalyst for the oxygen reduction reaction. One type of catalyst is nitrogen-doped graphene-oxide nanosheets. Graphene-oxide nanosheets are easily functionalized with other atoms such as boron, nitrogen, or sulfur, as well as metals such as iron, nickel, and cobalt, making them a versatile material for practical applications.
The process of making graphene-oxide nanosheets is done in an aqueous media and results in water molecules residing between the graphene sheets. Several researchers from Los Alamos National Laboratory, the University of New Mexico, Oak Ridge National Laboratory, and Rutgers University have characterized the effects of removing these intercalated water molecules. They found that the water molecules not only affect the nanosheets' physical structure and the concentration of heteroatoms that are added to it, but removal of the water molecules changes the nanosheets' catalytic activity. Their work appears in Science Advances.
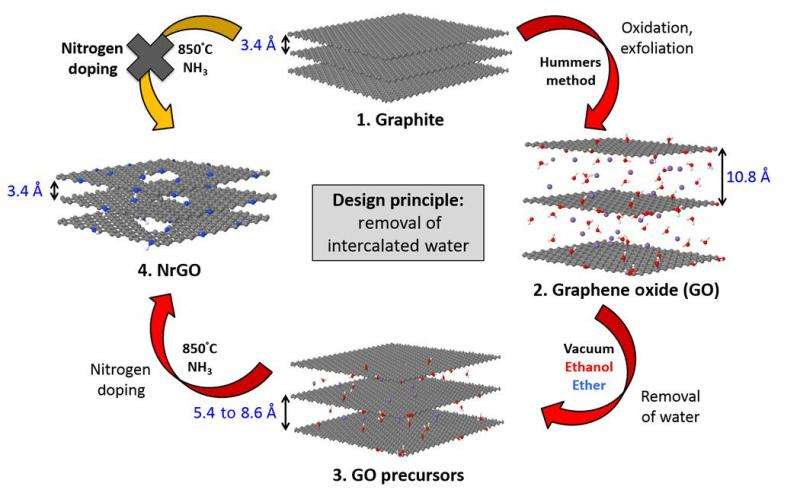
Graphite is oxidized to form graphene oxide sheets. These sheets typically have intercalated water molecules between the sheets. To make the catalyst, these sheets are reduced and doped with a heteroatom to form doped graphene sheets. Few studies have been conducted to understand the effects that the intercalated water molecules have on graphene-oxide nanosheets.
Using vacuum-drying and solvent washing with solvents that satisfy certain Hansen solubility parameters, Martinez, et al. were able to dry graphene-oxide sheets and remove the intercalated water molecules between the sheets. XRD studies verify that the intersheet distance decreased significantly after vacuum drying and then even more so after solvent drying. The intersheet distance changed from 10.8Å to 8.6Å after vacuum drying and then to 7.5Å after solvent drying with ethanol or diethyl ether. Furthermore, XRD data also showed evidence of long-range order.
XPS and IR data also verified that the sheets lacked intercalated water. Molecular dynamics studies confirmed that the solvents substantially alter the structure of water-intercalated graphene-oxide sheets. Notably the ether-treated sheets demonstrated a kind of "wrinkling" that may be partly why the ether-treated sheets displayed the best catalytic activity for the oxygen reduction reaction.
Nitrogen-doping was accomplished using a known method. Martinez, et al. treated the solvent-rinsed and vacuum-dried graphene oxide sheets with ammonia at a high temperature. They noted that the ethanol- and ether-treated sheets had large holes in the sheets, which is helpful for catalysis because it exposes interlayer active sites.
Martinez, et al. then tested their new nitrogen-doped reduced graphene-oxide catalyst (NrGO) with a rotating-ring disc electrode in acidic media. They looked for the three key features for a good catalyst: low overpotential, high half-wave potential, and selectivity for oxygen's four-electron reduction to water. The overpotential is the additional potential beyond the theoretical potential for an oxygen reduction reaction (Eo = 1.23V vs. RHE). Oxygen can undergo either a four-electron reduction reaction to produce water or a two-electron reduction reaction to form hydrogen peroxide. The more selective the catalyst is for the four-electron reaction, the better.
They found that the NrGO that was treated with diethyl ether showed the best overpotential, half-wave potential, and selectivity values compared to vacuum dried, ethanol-treated, and untreated graphene-oxide sheets. Martinez, et al. reported that this is the highest oxygen reduction reactivity to date for NrGO catalysts in acidic media.
This research provides valuable insight into how intercalated water affects the catalytic activity of graphene-oxide nanosheets. According to Dr. Gautam Gupta, principle investigator in this study, this study is important for proton exchange membrane fuel cells because they require acidic conditions. When asked about the implications of his research, Dr. Gupta said, "This is the first report that emphasizes the key role of water in catalysis and the research has significant implications in designing 2D materials such as transition metal dichalcogenides for energy applications."
More information: U. Martinez et al. Critical role of intercalated water for electrocatalytically active nitrogen-doped graphitic systems, Science Advances (2016). DOI: 10.1126/sciadv.1501178
Abstract
Graphitic materials are essential in energy conversion and storage because of their excellent chemical and electrical properties. The strategy for obtaining functional graphitic materials involves graphite oxidation and subsequent dissolution in aqueous media, forming graphene-oxide nanosheets (GNs). Restacked GNs contain substantial intercalated water that can react with heteroatom dopants or the graphene lattice during reduction. We demonstrate that removal of intercalated water using simple solvent treatments causes significant structural reorganization, substantially affecting the oxygen reduction reaction (ORR) activity and stability of nitrogen-doped graphitic systems. Amid contrasting reports describing the ORR activity of GN-based catalysts in alkaline electrolytes, we demonstrate superior activity in an acidic electrolyte with an onset potential of ~0.9 V, a half-wave potential (E½) of 0.71 V, and a selectivity for four-electron reduction of >95%. Further, durability testing showed E½ retention >95% in N2- and O2-saturated solutions after 2000 cycles, demonstrating the highest ORR activity and stability reported to date for GN-based electrocatalysts in acidic media.
Journal information: Science Advances
© 2016 Phys.org