December 1, 2015 report
Physicists confirm thermodynamic irreversibility in a quantum system
(Phys.org)—For the first time, physicists have performed an experiment confirming that thermodynamic processes are irreversible in a quantum system—meaning that, even on the quantum level, you can't put a broken egg back into its shell. The results have implications for understanding thermodynamics in quantum systems and, in turn, designing quantum computers and other quantum information technologies.
The physicists, Tiago Batalhão at the Federal University of ABC, Brazil, and coauthors, have published their paper on the experimental demonstration of quantum thermodynamic irreversibility in a recent issue of Physical Review Letters.
Irreversibility at the quantum level may seem obvious to most people because it matches our observations of the everyday, macroscopic world. However, it is not as straightforward to physicists because the microscopic laws of physics, such as the Schrödinger equation, are "time-symmetric," or reversible. In theory, forward and backward microscopic processes are indistinguishable.
In reality, however, we only observe forward processes, not reversible ones like broken egg shells being put back together. It's clear that, at the macroscopic level, the laws run counter to what we observe. Now the new study shows that the laws don't match what happens at the quantum level, either.
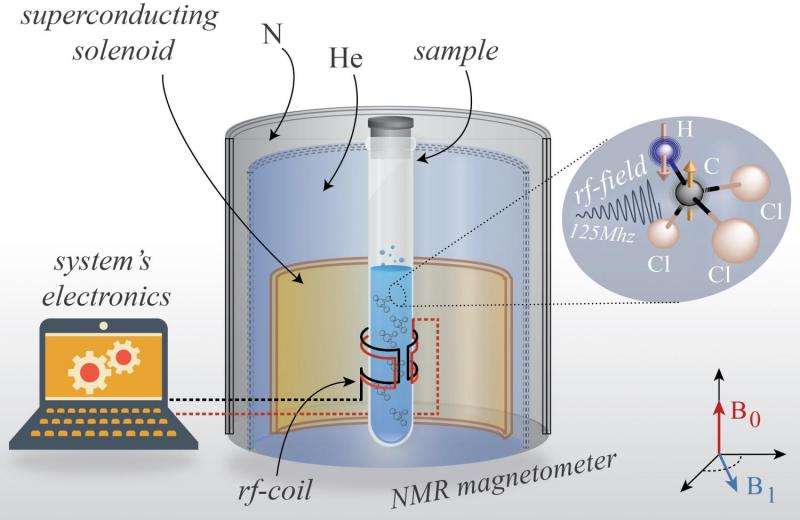
Observing thermodynamic processes in a quantum system is very difficult and has not been done until now. In their experiment, the scientists measured the entropy change that occurs when applying an oscillating magnetic field to carbon-13 atoms in liquid chloroform. They first applied a magnetic field pulse that causes the atoms' nuclear spins to flip, and then applied the pulse in reverse to make the spins undergo the reversed dynamics.
If the procedure were reversible, the spins would have returned to their starting points—but they didn't. Basically, the forward and reverse magnetic pulses were applied so rapidly that the spins' flipping couldn't always keep up, so the spins were driven out of equilibrium. The measurements of the spins indicated that entropy was increasing in the isolated system, showing that the quantum thermodynamic process was irreversible.
By demonstrating that thermodynamic irreversibility occurs even at the quantum level, the results reveal that thermodynamic irreversibility emerges at a genuine microscopic scale. This finding makes the question of why the microscopic laws of physics don't match our observations even more pressing. If the laws really are reversible, then what are the physical origins of the time-asymmetric entropy production that we observe?
The physicists explain that the answer to this question lies in the choice of the initial conditions. The microscopic laws allow reversible processes only because they begin with "a genuine equilibrium process for which the entropy production vanishes at all times," the scientists write in their paper. Preparing such an ideal initial state in a physical system is extremely complex, and the initial states of all observed processes aren't at "genuine equilibrium," which is why they lead to irreversible processes.
"Our experiment shows the irreversible nature of quantum dynamics, but does not pinpoint, experimentally, what causes it at the microscopic level, what determines the onset of the arrow of time," coauthor Mauro Paternostro at Queen's University in Belfast, UK, told Phys.org. "Addressing it would clarify the ultimate reason for its emergence."
The researchers hope to apply the new understanding of thermodynamics at the quantum level to high-performance quantum technologies in the future.
"Any progress towards the management of finite-time thermodynamic processes at the quantum level is a step forward towards the realization of a fully fledged thermo-machine that can exploit the laws of quantum mechanics to overcome the performance limitations of classical devices," Paternostro said. "This work shows the implications for reversibility (or lack thereof) of non-equilibrium quantum dynamics. Once we characterize it, we can harness it at the technological level."
More information: T. B. Batalhão, et al. "Irreversibility and the Arrow of Time in a Quenched Quantum System." Physical Review Letters. DOI: 10.1103/PhysRevLett.115.190601
Also at arXiv:1502.06704 [quant-ph]
Journal information: Physical Review Letters
© 2015 Phys.org