How does titanium oxide promote water oxidation in hematite-based photoanodes?
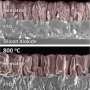
Hematite is an expensive, Earth-abundant photoactive material that can be used as a photoanode for water splitting devices. It offers attractive properties, such as a low band gap that allows it to theoretically satisfy the requirements for implementing practical photoelectrochemical systems with a life-cycle net energy feasible for large-scale hydrogen production facilities. It also has a set of intrinsic limitations that lessen its maximum photooxidation performance. Among the methods used for improving its photoactivity, titanium doping has witnessed an intensive research during recent years.
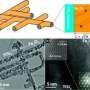
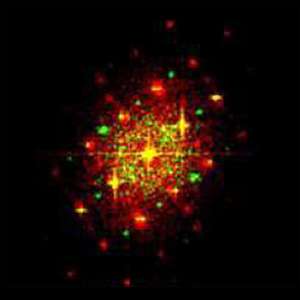
Water splitting is a chemical reaction in which water is separated into oxygen and hydrogen. Obtaining hydrogen from a cheap and abundant source such as water may have broad energetic implications. A work recently published in Energy & Environmental Science, led by the Catalonia Institute for Energy Research (IREC) with the participation of the Advanced Electron Nanoscopy Group leaded by ICREA Prof Jordi Arbiol at the ICN2, analyses the origin of the titanium-induced enhanced photoactivity in hematite-based photoanodes. The researchers prepared mesoporous hematite (host) / titania (guest) composite films and performed voltammetric and impedance measurements, assessed the roles of surface states and charge donor (dopant) densities. To understand the photooxidation mechanism, ICN2 researchers modeled the atomic scale morphology of the used complex core-shell nanomaterials composing the Hematite based photoanodes by using HRTEM and EELS measurements.
The results revealed that a detailed balance between surface states, doping levels, heterojunction formation and phase solubility is required to obtain the optimum photocurrent from titanium doped hematite photoanodes. From a wider perspective, this work provides a solid basis for understanding the interplay of the different actors involved in the photoactivity improvement of a host-guest composite, up to the solubility limit of its components.
More information: "What Do you Do, Titanium? Insight into the Role of Titanium Oxide as Water Oxidation Promoter in Hematite-based Photoanodes." Energy Environ. Sci., 2015, Accepted Manuscript DOI: 10.1039/C5EE01679G
Journal information: Energy & Environmental Science