From cameras to computers, new material could change how we work and play
Serendipity has as much a place in science as in love. That's what Northeastern physicists Swastik Kar and Srinivas Sridhar found during their four-year project to modify graphene, a stronger-than-steel infinitesimally thin lattice of tightly packed carbon atoms. Primarily funded by the Army Research Laboratory and Defense Advanced Research Projects Agency, or DARPA, the researchers were charged with imbuing the decade-old material with thermal sensitivity for use in infrared imaging devices such as night-vision goggles for the military.
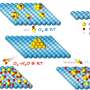
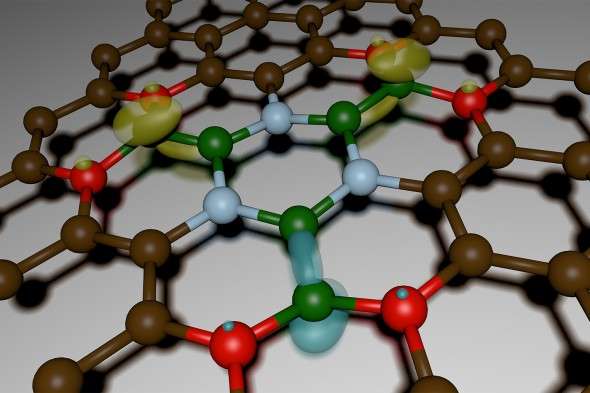
What they unearthed, published Friday in the journal Science Advances, was so much more: an entirely new material spun out of boron, nitrogen, carbon, and oxygen that shows evidence of magnetic, optical, and electrical properties as well as DARPA's sought-after thermal ones. Its potential applications run the gamut: from 20-megapixel arrays for cellphone cameras to photo detectors to atomically thin transistors that when multiplied by the billions could fuel computers.
"We had to start from scratch and build everything," says Kar, an assistant professor of physics in the College of Science. "We were on a journey, creating a new path, a new direction of research."
The pair was familiar with "alloys," controlled combinations of elements that resulted in materials with properties that surpassed graphene's—for example, the addition of boron and nitrogen to graphene's carbon to connote the conductivity necessary to produce an electrical insulator. But no one had ever thought of choosing oxygen to add to the mix.
What led the Northeastern researchers to do so?
"Well, we didn't choose oxygen," says Kar, smiling broadly. "Oxygen chose us."
Oxygen, of course, is everywhere. Indeed, Kar and Sridhar spent a lot of time trying to get rid of the oxygen seeping into their brew, worried that it would contaminate the "pure" material they were seeking to develop.
"That's where the Aha! moment happened for us," says Kar. "We realized we could not ignore the role that oxygen plays in the way these elements mix together."
"So instead of trying to remove oxygen, we thought: Let's control its introduction," adds Sridhar, the Arts and Sciences Distinguished Professor of Physics and director of Northeastern's Electronic Materials Research Institute.
Oxygen, it turned out, was behaving in the reaction chamber in a way the scientists had never anticipated: It was determining how the other elements—the boron, carbon, and nitrogen—combined in a solid, crystal form, while also inserting itself into the lattice. The trace amounts of oxygen were, metaphorically, "etching away" some of the patches of carbon, explains Kar, making room for the boron and nitrogen to fill the gaps.
"It was as if the oxygen was controlling the geometric structure," says Sridhar.
They named the new material, sensibly, 2D-BNCO, representing the four elements in the mix and the two-dimensionality of the super-thin lightweight material, and set about characterizing and manufacturing it, to ensure it was both reproducible and scalable. That meant investigating the myriad permutations of the four ingredients, holding three constant while varying the measurement of the remaining one, and vice versa, multiple times over.
After each trial, they analyzed the structure and the functional properties of the product— electrical, optical—using electron microscopes and spectroscopic tools, and collaborated with computational physicists, who created models of the structures to see if the configurations would be feasible in the real world.
Next they will examine the new material's mechanical properties and begin to experimentally validate the magnetic ones conferred, surprisingly, by the intermingling of these four nonmagnetic elements. "You begin to see very quickly how complicated that process is," says Kar.
Helping with that complexity were collaborators from around the globe. In addition to Northeastern associate research scientists, postdoctoral fellows, and graduate students, contributors included researchers in government, industry, and academia from the United States, Mexico, and India.
"There is still a long way to go but there are clear indications that we can tune the electrical properties of these materials," says Sridhar. "And if we find the right combination, we will very likely get to that point where we reach the thermal sensitivity that DARPA was initially looking for as well as many as-yet unforeseen applications."
More information: Science Advances 31 Jul 2015: Vol. 1, no. 6, e1500094. DOI: 10.1126/sciadv.1500094
Journal information: Science Advances
Provided by Northeastern University