May 22, 2015 feature
Semiliquid battery competitive with both Li-ion batteries and supercapacitors
(Phys.org)—A new semiliquid battery developed by researchers at The University of Texas at Austin has exhibited encouraging early results, encompassing many of the features desired in a state-of-the-art energy-storage device. In particular, the new battery has a working voltage similar to that of a lithium-ion battery, a power density comparable to that of a supercapacitor, and it can maintain its good performance even when being charged and discharged at very high rates.
The researchers, led by Assistant Professor Guihua Yu, along with Yu Ding and Yu Zhao, at UT Austin, have published their paper on the new membrane-free, semiliquid battery in a recent issue of Nano Letters. The researchers explain that the battery is considered "semiliquid" because it uses a liquid ferrocene electrolyte, a liquid cathode, and a solid lithium anode.
"The greatest significance of our work is that we have designed a semiliquid battery based on a new chemistry," Yu told Phys.org. "The battery shows excellent rate capability that can be fully charged or discharged almost within one minute while maintaining good energy efficiency and reasonable energy density, representing a promising prototype liquid redox battery with both high energy density and power density for energy storage."
The battery is designed for applications in two of the biggest areas of battery technology: hybrid electric vehicles and energy storage for renewable energy resources.
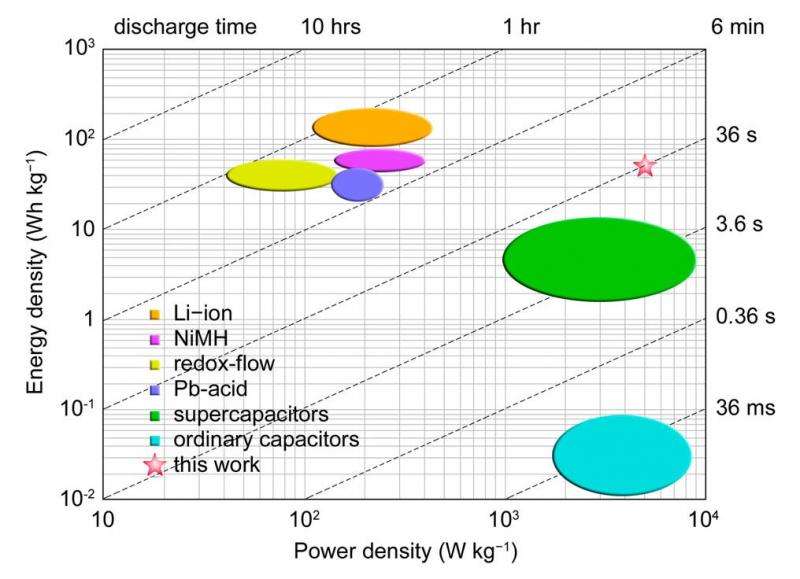
As shown in the figure above, the battery's high power density (1400 W/L) and good energy density (40 Wh/L) put it in the uniquely favorable position of combining a power density that is as high as that of current supercapacitors with an energy density on par with those of state-of-the-art redox flow batteries and lead-acid batteries, though slightly lower than that of lithium-ion batteries. This combination is especially attractive for electric vehicles, where the power density corresponds to top speed and the energy density to the vehicle's range per charge.
The researchers also report in their paper that the new battery has a high capacity (137 mAh/g) and a high capacity retention of 80% for 500 cycles.
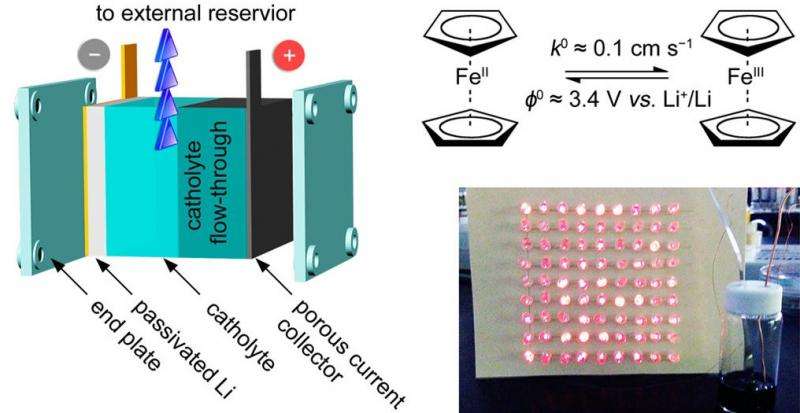
The researchers attribute the battery's good performance in large part to its liquid electrode design that enables its high rate capability, which is basically a measure of how fast the battery operates. The ions can move through the liquid battery very rapidly compared to in a solid battery, and the redox reactions in which the electrons are transferred between electrodes also occur at very high rates in this particular battery. For comparison, the values used to measure these rates (the diffusion coefficient and the reaction constant) are orders of magnitude greater in the new battery than in most conventional flow batteries.
Although the battery looks very promising so far, the researchers note that more work still needs to be done, in particular regarding the lithium anode.
"The potential weakness of this battery is the lithium anode in terms of long-term stability and safety," Yu said. "More advanced lithium anode protection is required to fully suppress self-discharge. We suppose that other metals like zinc and magnesium may also function as the anode for such a battery as long as the electrolyte compatibility is resolved. We also expect that other organometallic compounds with multi-valence-state metal centers (redox centers) may also function as the anode, which eventually would make the battery fully liquid."
In the future, the researchers plan to test the long-term durability of the battery, especially its lithium anode, under realistic operating conditions. In addition, the researchers want to find a way to increase the solubility of ferrocene in order to further increase the energy density to compete with current lithium-ion batteries while maintaining its very high power density.
More information: Yu Ding, et al. "A Membrane-Free Ferrocene-Based High-Rate Semiliquid Battery." Nano Letters. DOI: 10.1021/acs.nanolett.5b01224
Journal information: Nano Letters
© 2015 Phys.org