March 26, 2015 feature
Now you see it: Real-space observation of many-body proton tunneling in water nanocluster
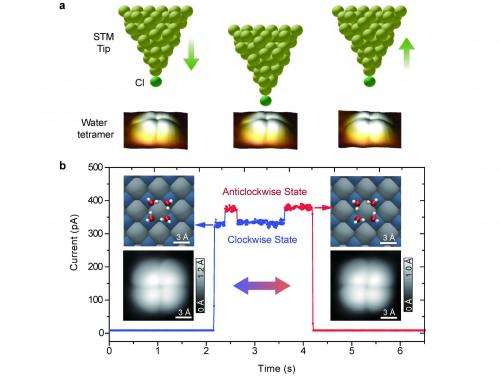
There's more to quantum tunneling than meets the eye – or rather, the visualization technique. (Quantum tunneling is a quantum mechanical phenomenon where a particle transitions through a classically-forbidden energy state.) Most quantum tunneling discussion focus on incoherent single-particle tunneling; on the other hand, quantum tunneling in the context of proton dynamics usually involves many hydrogen bonds at once, which leads to what is known as correlated many-body tunneling. (The many-body problem refers to the properties of microscopic systems that are described by quantum mechanics, comprising a large number of interacting particles – that is, ≥ 3 – which can become entangled.) The downside is that while single-particle tunneling is well understood, many-body tunneling is still shrouded in mystery. Recently, however, scientists at Peking University, Beijing reported the real-space observation of concerted proton tunneling in a cyclic water tetramer – a macromolecular nanocluster consisting of four water molecules arranged in a loop or ring – by using a cryogenic scanning tunneling microscope (STM). The scientists found that the presence of the Cl- chlorine anion (a negatively charged chlorine ion) at the STM tip apex may either enhance or suppress the concerted tunneling process based on the coupling symmetry between the ion and the protons, adding that their work may allow the control the quantum states of protons with atomic-scale precision.
Prof. Ying Jiang discussed the paper that he, Prof. En-Ge Wang and their colleagues published in Nature Physics, noting that one of the main challenges they encountered was directly visualizing the concerted tunneling of four protons in an individual hydrogen-bonded water tetramer adsorbed on a gold-supported halite film. "One basic requirement is to locate in real space the position of protons within the hydrogen-bonded network, such that the motion of the protons can be tracked," Jiang tells Phys.org. "This is extremely difficult for any microscopes due to the light mass and small size of protons – and even worse is that the travelling distance of the protons across the hydrogen bonds is less than one ångström (10-10m). As a result, attacking this problem ideally requires the ability to access the internal degree of freedom of water molecule. Fortunately, we developed a novel submolecular imaging technique last year1 that allows us to discriminate the orientation of water molecules as well as the hydrogen-bonding directionality." This technique is what paved the way for the scientists to address the proton dynamics within the hydrogen-bonded network.
"Furthermore," Jiang continues, "the concerted tunneling or many-body correlated tunneling of the protons is extremely sensitive to the atomic-scale environment, and can be easily disturbed or even killed by the probes." This is due to the fact that the concerted tunneling of protons is a coherent quantum process, which requires all the protons to have exactly the same phase – and the asymmetric coupling between the probes and the protons can destroy the phase correlation between the protons and quench the collective tunneling. "In other words, one may easily mess up such concerted tunneling process just because the STM tip is not in the correct position. Therefore, we need to position the STM tip very precisely inside the water tetramer to ensure the symmetric coupling, where the four protons are all equally coupled with the STM tip." To search for such a precise position requires a great deal of care and patience: If the STM tip is off by only 10 picometers (10-12m), the researchers may obtain completely different results.
Another issue was finding that the presence of the chlorine anion at the STM tip apex may either enhance or suppress the concerted tunneling process, depending on the details of the coupling symmetry between the Cl anion and the protons. "I have to emphasize that it is far more difficult to control the concerted tunneling of protons than to simply visualize this process – it means that one has to simultaneously manipulate several quantum particles in real space." That is, while the STM tip not only acts as a local probe, but can be used to manipulate the single atoms or molecules on surface with atomic-scale precision, manipulating many-body states is challenging. "It's essential to always keep the coupling geometry between the STM tip and four protons in a symmetric way during the manipulation," Jiang points out. "Otherwise, the concerted tunneling is easily suppressed or even quenched."
In addition, Jiang continues, the Cl anion at the tip apex is very important for achieving the efficient manipulation of the concerted proton tunneling. "To be honest, we actually learned this from an accident: For months, we tried to control the concerted tunneling process with a bare metal tip, but all attempts failed. One day the tip was crashed into the gold-supported sodium chloride film substrate due to a faulty operation. Unexpectedly, using this 'bad' tip, we were able to enhance the tunneling rate very efficiently." The scientists later determined that this occurred because the tip picked up a chlorine atom from the sodium chloride surface – and since the chlorine atom is electronegative in nature, the tip is negatively charged. The long-range electric interaction between the negatively charged chlorine atom and the positively charged protons then leads to the suppression of the tunneling barrier.
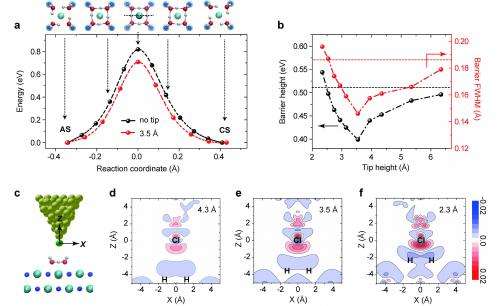
"Without the Cl- tip, the proton has to travel for a long distance from one water molecule to the other, and the energy barrier is quite high. Inserting a chlorine anion between the water molecules establishes a 'bridge' for the protons. The attraction of Cl- assists the protons, as it were, and thereby assists the proton transfer process," Jiang explains, "That's the physical analogy of why the energy barrier is suppressed by the tip/proton coupling."
After this accidental tip crashing, the researchers invested quite some time to explore a controllable and reproducible way to functionalize the tip apex with a single chlorine atom. "We discovered that chlorine atoms on the sodium chloride surface seemed very 'tip-friendly.'" Once they manipulated a bare tip to closely approach the NaCl(001) surface – that is, one in which crystalline cleavage occurs parallel to the faces of a cube – and then applied the proper voltage pulse, the chlorine atom readily translocated onto the tip end and became very stable.
Moreover, further lowering of the tip height leads to continuous decrease of the barrier because the electric interaction gets stronger – and if the tunneling barrier can be suppressed to such an extent that the zero-point energy of the protons exceeds the barrier height, an extreme quantum effect – that is, complete quantum delocalization – may occur. "In such a case," Jiang notes, "the protons are shared by two nearest-neighboring water molecules, and the originally asymmetric hydrogen bond then becomes symmetric. This is a much stronger quantum effect than quantum tunneling, which we are still struggling to explore."
A third challenge was tuning the Cl-/proton electric coupling in three dimensions with picometer precision. "It's no exaggeration to say that tuning the coupling of protons to the atomic-scale environment in three dimensions with picometer precision is not possible with any technique other than STM. With the combination of the tip height z and tip lateral position x, y, we can actually achieve any coupling geometry between the Cl anion and the protons." Due to the high stability of their STM, the precision for tuning the dimensions can get down to one picometer or better, which is essential for controlling the many-body quantum states of protons. "We were very surprised to see that 10 picometer change in the tip height (z direction) can lead to almost one order of magnitude difference in the tunneling rate. This again shows the extreme sensitivity of the many-body tunneling to the atomic-scale environment, which has never been observed before."
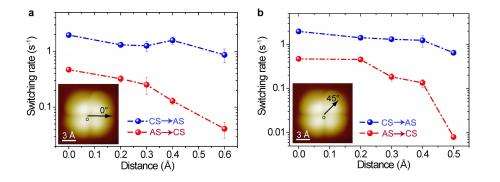
The paper details how the scientists explored the role of individual chlorine anions in influencing the correlated tunneling process by using the Cl--terminated tip, which if located at the exact center of the water tetramer, the Cl anion on the tip apex is equally coupled with the four protons and the cooperativity of the protons is reserved. (Cooperativity is a phenomenon displayed by systems involving identical or near-identical elements, which act non-independently of each other, relative to a hypothetical standard non-interacting system in which the individual elements are acting independently.) "The tunneling probability can be greatly enhanced by the Cl-/proton electric attraction – but if the tip is slightly moved off the center at, for example, the picometer scale, asymmetric coupling occurs. If that occurs, even if the Cl-/proton electric attraction is still present, the phase coherence between the protons can be easily destroyed due to inequivalent coupling between the protons and the chlorine anion. In such a case, the four protons can hardly move at the same pace and one would expect a rapid quenching of the correlated tunneling process."
When asked about the significant implications and potential applications of controlling the quantum states of protons with atomic-scale precision as made possible by their work, Jiang told Phys.org that the ability to control the quantum states of protons "can certainly improve our understanding of the role of quantum mechanics in proton dynamics, such as phase transition in ices of high-pressure phases. It may also provide completely new routes for the design of new energy, new medicine and new functional materials related to proton transfer."
Jiang adds that a less straightforward but very ambitious application is quantum computing. "The two many-body states of the four protons can be adopted to build a qubit, which is essential in quantum computing. If there is a way to decouple the water tetramer from the environment, we should be able to observe the superposition of the two many-body states. However, the biggest challenge lies in how to realize coherent control on and readout of the two many-body states. Since scattering by tunneling electrons from the STM tip tends to destroy the quantum coherence of protons, it seems that we need to develop new techniques other than STM to realize such control."
Moving forward with their research, the scientists are now trying to build larger hydrogen-bonded water clusters on substrates to explore more novel correlated quantum behaviors of protons. "We're also curious about the upper limit of the number of protons at which cooperativity and tunnel collectively" – that is, correlated many-body tunneling – "can be maintained. Another thing we're planning to do is using an accurately-engineered STM tip to further suppress the tunneling barrier such that the zero-point motion of protons can surpass the energy barrier. We then expect to visualize the complete quantum delocalization at single proton level."
One innovation the researchers are interested in developing is achieving coherent control on the many-body quantum states of protons, as described above; another is improving the temporal resolution of their STM system, such that they can closely follow the coherent evolution of the many-body states in real time. "These new techniques may well make it possible to observe the Rabi oscillation of proton states, which is a common phenomenon for photon- or spin-based two-level systems." The Rabi oscillation, or Rabi cycle, is the cyclic behavior of a two-state (with non-equal energies) quantum system important in quantum optics, nuclear magnetic resonance and quantum computing that, in the presence of an oscillatory driving field, can become excited when it absorbs a quantum of energy.
As to other areas of research that might benefit from the study, Jiang tells Phys.org that "the improved understanding and the real-space control of correlated proton tunneling may have great impact in an extremely broad spectrum of research fields, such as phase transition, signal transduction, topological organic ferroelectrics, photosynthesis, and enzyme catalysis, to name just a few."
More information: Direct visualization of concerted proton tunnelling in a water nanocluster, Nature Physics (2015) 11:235–239, doi:10.1038/nphys3225
Related:
1Real-space imaging of interfacial water with submolecular resolution, Nature Materials (2014) 13:184–189, doi:10.1038/nmat3848
Journal information: Nature Physics , Nature Materials
© 2015 Phys.org





















